Creating effective diagnostic tools from waste materials might sound like science fiction, but researchers have developed a COVID-19 biosensor built from recycled battery graphite and plastic cups. This innovative device detects SARS-CoV-2 proteins in saliva samples with accuracy comparable to standard PCR tests, while costing just 20 cents per test.
The device combines two key technologies: magnetic nanoparticles that capture viral proteins, and a 3D-printed electrochemical sensor that measures the capture event. Unlike conventional tests that require expensive laboratory equipment, this biosensor can work in basic healthcare settings.
Most remarkably, it's built almost entirely from materials that would otherwise end up in landfills, demonstrating how circular economy principles can create sustainable healthcare solutions.
Key Takeaways
- A 3D-printed testing device uses recycled graphite from old batteries and plastic from disposable cups as its core components.
- Magnetic nanoparticles, modified with COVID-19-specific antibodies, capture viral proteins from saliva samples in just 15 minutes.
- The biosensor detects incredibly small amounts of viral protein - as little as 3.46 picograms per milliliter (equivalent to detecting a grain of salt in an Olympic swimming pool).
- When tested on 60 real saliva samples, the device achieved 86.7% sensitivity and 97.8% specificity compared to PCR tests.
- Each test costs approximately 20 cents to manufacture, making it viable for mass testing programs.
- The device's modular design means it could be adapted to detect other diseases by simply changing the capture antibodies.
Figure 1. Low-cost biosensors made from recycled materials (a) Schematic representation for preparation of the 3D-printed magneto-integrated electrochemical device (MED) in 3-steps. Credit: Carvalho et al.
How The Technology Works
The biosensor's ingenious design starts with waste recovery. Researchers extract graphite rods from discarded zinc-carbon batteries (the kind found in TV remotes and flashlights) and dissolve polystyrene from disposable coffee cups. These materials are combined to create a conductive ink that forms the heart of the sensor.
Using a standard 3D printer, the team creates a tiny three-electrode testing chamber about the size of a coin. The graphite-plastic mixture is applied to create conductive pathways, and a small magnet is embedded beneath the working electrode. After mechanical polishing and chemical treatment with sodium hydroxide, the electrodes become highly responsive to electrical signals.
The magnetic capture system uses iron-manganese oxide nanoparticles, each about 15-16 nanometers across (roughly 5,000 times smaller than the width of human hair). These particles are chemically linked to antibodies that specifically recognize the spike protein of SARS-CoV-2. When mixed with a saliva sample, any viral proteins present bind to these magnetic antibody-particle complexes.
Detection happens through a clever electrical measurement. The magnet pulls the nanoparticles (with any bound viral proteins) onto the electrode surface. When an electrical current passes through the system, bound viral proteins act like tiny roadblocks, reducing the current flow. The more virus present, the greater the current reduction, allowing precise quantification of viral load.
Why This Innovation Matters
The COVID-19 pandemic highlighted critical gaps in global diagnostic capacity, particularly in low-resource settings where expensive laboratory infrastructure is unavailable. While PCR testing remains the gold standard, it requires sophisticated equipment, trained technicians, and stable supply chains for reagents. This biosensor addresses these limitations by offering comparable accuracy at a fraction of the cost using materials available almost anywhere.
Beyond immediate pandemic response, this technology demonstrates important progress toward sustainable healthcare innovation. The circular economy approach transforms waste products into valuable medical tools, potentially diverting millions of batteries and plastic cups from landfills while addressing public health needs. If scaled globally, such approaches could significantly reduce both healthcare costs and environmental impact.
The modular design opens doors to broader applications. By switching antibodies, researchers could adapt the same platform to detect dengue fever, HIV, malaria, or emerging pandemic threats. This flexibility makes it particularly valuable for surveillance programs in regions where multiple infectious diseases circulate simultaneously.
From a technical standpoint, the work advances several fields simultaneously. It provides a practical protocol for recycling electronic waste into functional sensors, demonstrates effective magnetic separation in complex biological samples, and validates label-free electrochemical detection in real clinical specimens. These contributions could inspire similar innovations across diagnostic medicine ((Crapnell and Banks, 2023); (Kalinke et al., 2024)).
Performance And Validation
Laboratory optimization. The researchers first needed to make their recycled materials work as effective sensors. Raw graphite-plastic electrodes showed poor electrical response because the plastic encapsulated the conductive graphite particles. However, mechanical polishing exposed the graphite, and chemical treatment with sodium hydroxide dramatically improved performance by creating oxygen-containing groups on the graphite surface that enhanced electrical conductivity.
Magnetic nanoparticle characterization. Electron microscopy confirmed that the iron-manganese oxide particles maintained their crystalline structure and uniform size after antibody attachment. Importantly, the particles retained their magnetic properties, allowing rapid collection by the embedded magnet while avoiding clumping that could interfere with binding.
Sensor response optimization. The team systematically tested different antibody concentrations and incubation times to maximize sensitivity while minimizing costs. They found that 20 micrograms per milliliter of antibody and 15-minute incubation provided the best balance, achieving detection limits in the low picogram range.
Clinical validation results. Testing on 60 heat-inactivated saliva samples from COVID-19 patients and healthy controls yielded impressive results. The biosensor correctly identified 86.7% of positive samples (sensitivity) and 97.8% of negative samples (specificity), with overall diagnostic accuracy of 95% compared to PCR testing. The few misclassifications likely resulted from protein damage during the heat inactivation process used for safety.
Interference testing. To ensure reliability in real-world conditions, researchers tested the sensor's response to potentially interfering substances commonly found in saliva, including other antibodies and proteins from dengue fever. The sensor showed excellent specificity, responding only to COVID-19 proteins and not to these potential interferents.
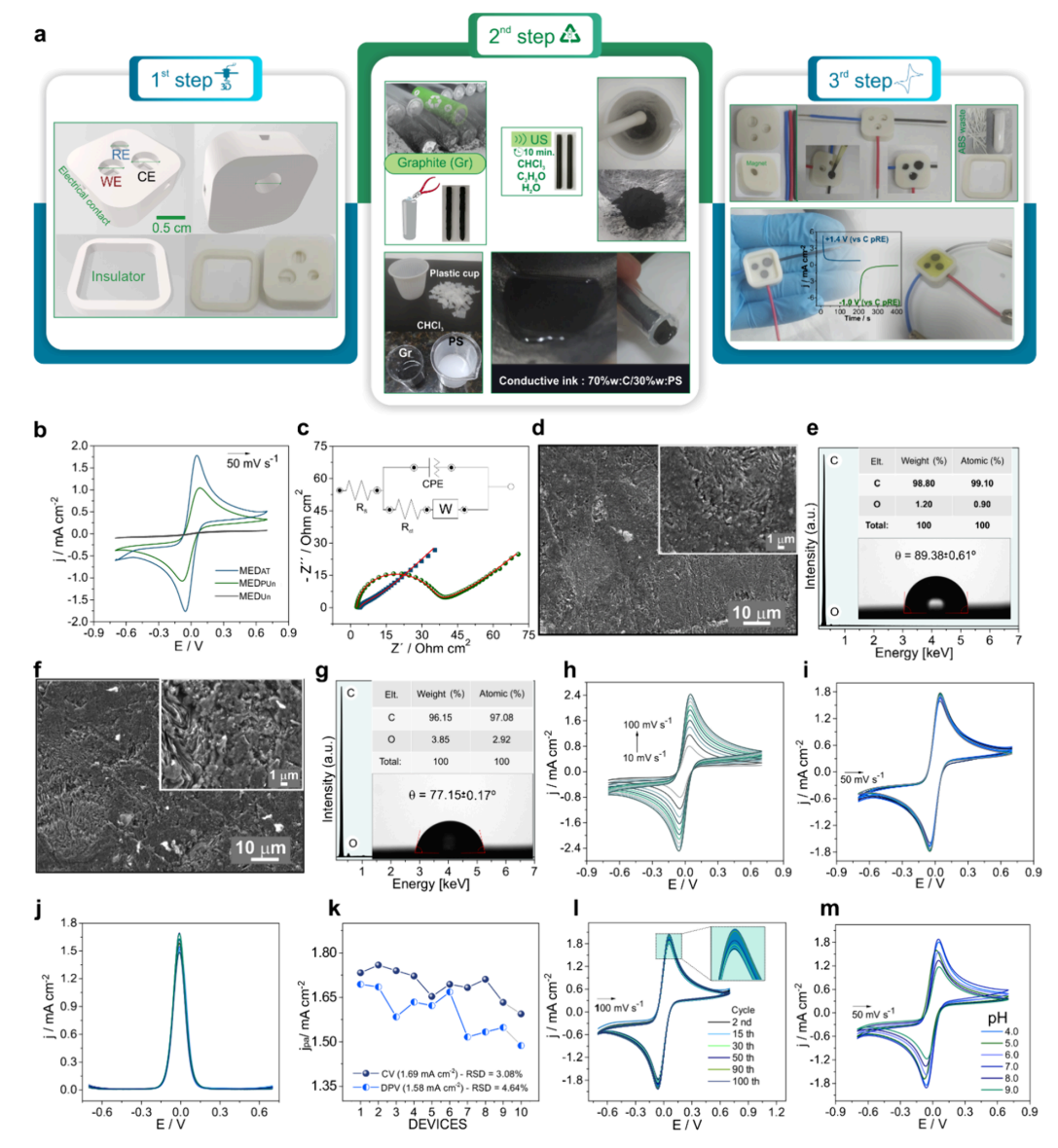
Device fabrication and optimization (Figure 1). The researchers systematically optimized their recycled electrode design through a three-step process. Figure 1 demonstrates how raw graphite-plastic electrodes initially showed poor electrical response (panel b, blue curve) because plastic encapsulated the conductive graphite particles. Mechanical polishing dramatically improved the electrode response by exposing graphite surfaces (red curve), while subsequent chemical treatment with sodium hydroxide created the optimal performance (green curve) by generating oxygen-containing surface groups that enhanced electrical conductivity. The electrochemical impedance measurements (panel c) confirmed these improvements, showing charge-transfer resistance dropping from over 1000 ohms to just 15 ohms after treatment. The device also demonstrated excellent reproducibility across different manufacturing batches and maintained stable performance over 100 measurement cycles, proving its robustness for practical applications.
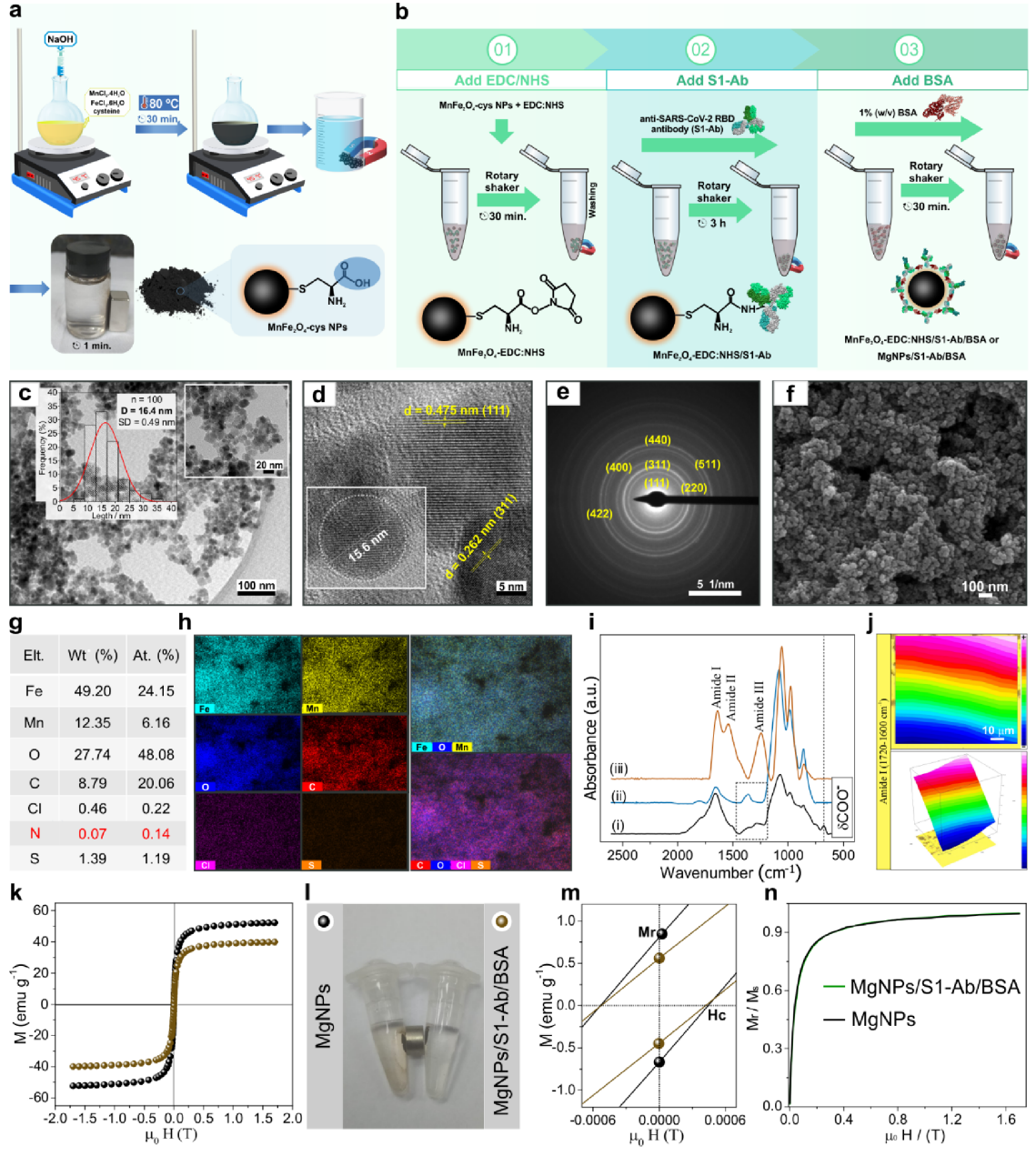
Magnetic nanoparticle development (Figure 2). Figure 2 reveals the sophisticated engineering behind the magnetic capture system. Transmission electron microscopy (panels c-d) confirmed the iron-manganese oxide particles maintained uniform 15-16 nanometer size and crystalline structure throughout the antibody attachment process. The infrared spectroscopy results (panel i) showed clear chemical signatures proving successful antibody conjugation through characteristic amide bonds. Most critically, magnetization measurements (panels k-m) demonstrated that while antibody attachment slightly reduced magnetic strength from 48.8 to 39.1 electromagnetic units per gram, the particles retained their superparamagnetic properties essential for rapid magnetic collection without permanent clumping. Visual demonstrations (panel l) showed the bioconjugated particles could be completely captured by a small magnet within seconds, validating the capture mechanism.
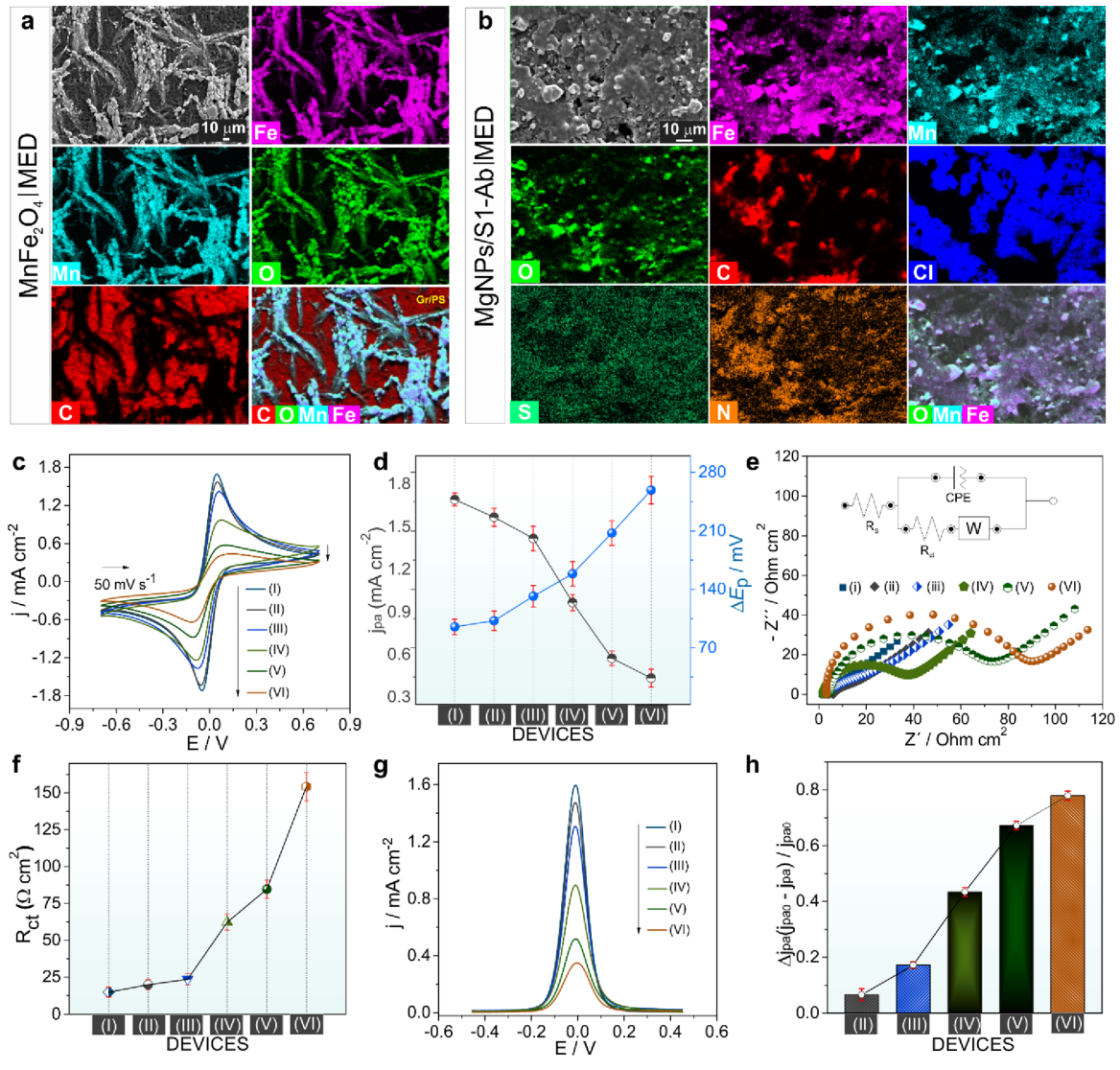
Biosensor interface characterization (Figure 3). Figure 3 systematically tracks the electrical changes as each component is added to build the complete biosensor. Each modification step - from bare electrode to magnetic particles, antibody attachment, protein blocking, and finally viral protein capture - produced predictable decreases in electrical current (panels c, g) and increases in electrical resistance (panels e, f). This stepwise progression confirmed proper assembly and provided a clear electrical signature for viral protein detection. The energy-dispersive X-ray mapping (panels a-b) visually confirmed uniform distribution of all components across the electrode surface, ensuring consistent sensor performance.
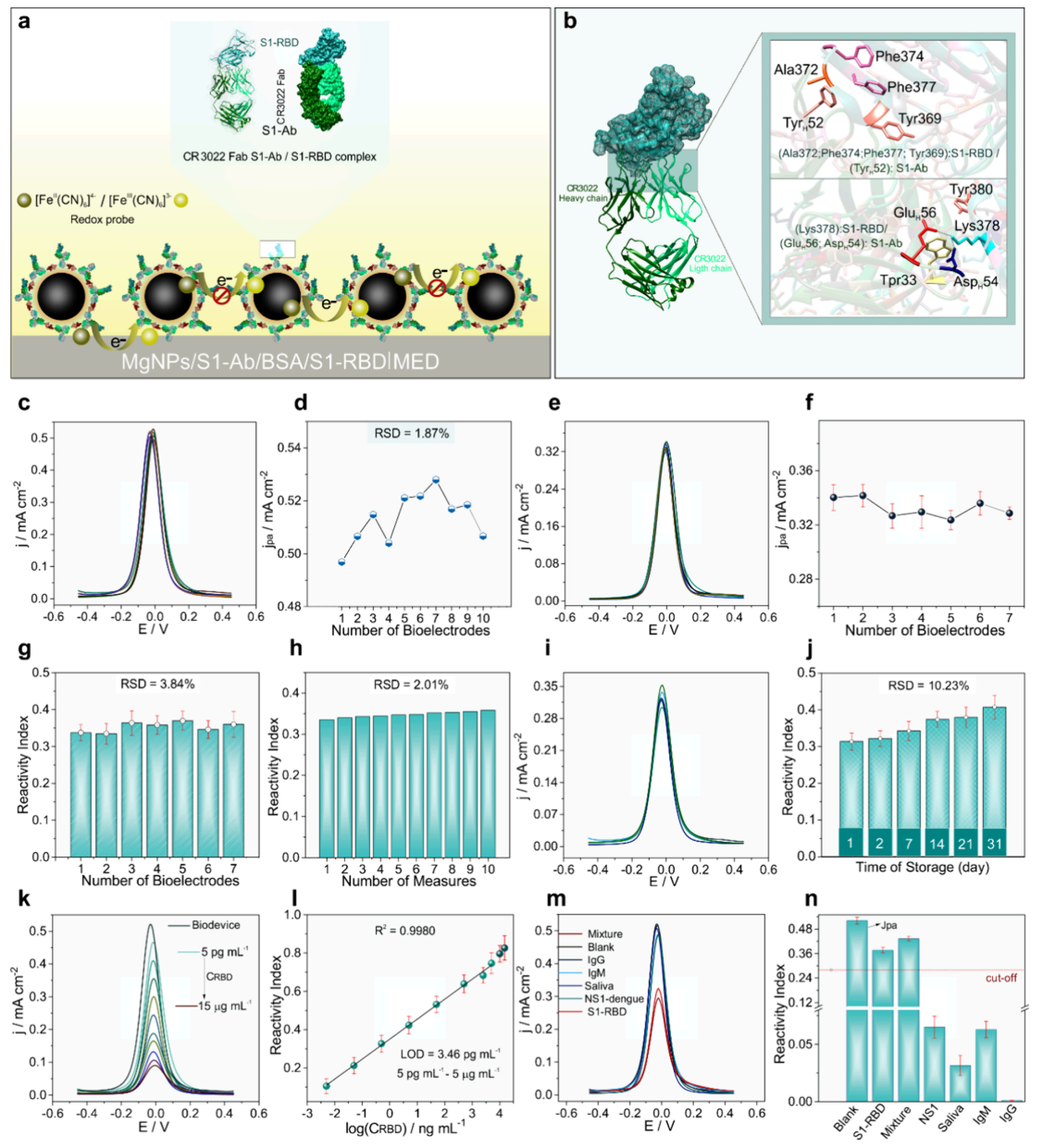
Analytical performance and interference testing (Figure 4). Figure 4 establishes the sensor's impressive analytical capabilities. The calibration curve (panel l) showed linear response across five orders of magnitude, from 5 picograms to 5 micrograms per milliliter, with a detection limit of 3.46 picograms per milliliter - sensitive enough to detect trace amounts of viral protein. Inter-device reproducibility testing (panels e-h) demonstrated remarkably consistent performance across multiple sensors, with relative standard deviations below 4%. Long-term stability studies (panels i-j) showed the biosensor maintained reliable performance for 30 days when stored at 4°C. Crucially, cross-reactivity testing (panels m-n) confirmed the sensor responded specifically to COVID-19 proteins and showed no interference from dengue fever proteins, human antibodies, or saliva components, proving its selectivity in complex biological samples.
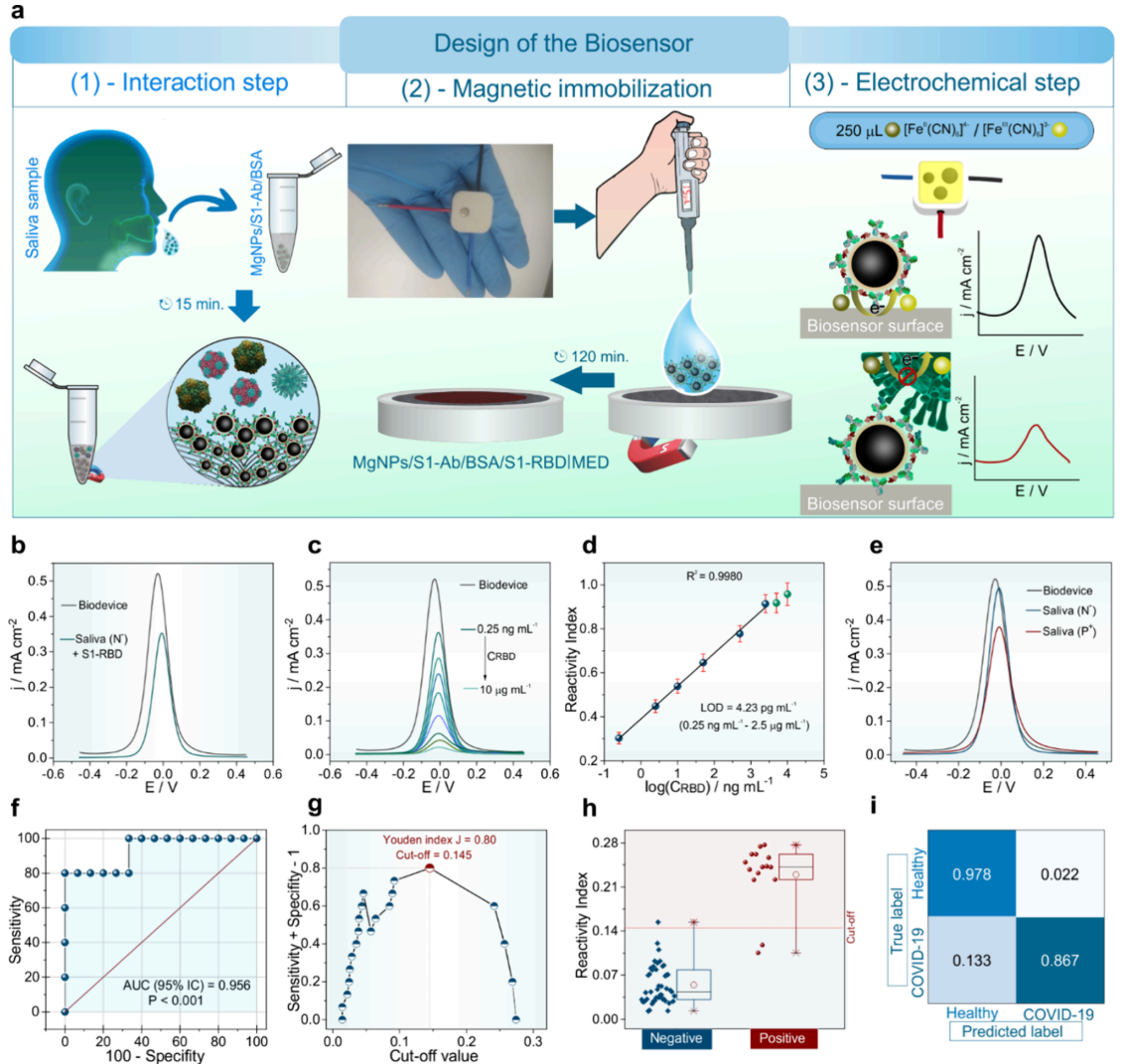
Clinical validation in human saliva (Figure 5). Figure 5 presents the decisive clinical validation using 60 real saliva samples from COVID-19 patients and healthy individuals. The receiver operating characteristic curve (panel f) achieved an area under the curve of 0.956, indicating excellent diagnostic performance. Using the statistically optimized cutoff value (panel g), the biosensor achieved 86.7% sensitivity and 97.8% specificity compared to PCR testing. The confusion matrix (panel i) revealed only 2 false negatives and 1 false positive out of 60 samples, yielding 95% overall accuracy. Box plot analysis (panel h) showed clear separation between positive and negative samples, while calibration in saliva (panel d) maintained the low detection limit of 4.23 picograms per milliliter with excellent recovery rates of 96-105%, proving the sensor works reliably in complex biological matrices.
Current Limitations And Future Prospects
While promising, the technology faces several practical challenges before widespread deployment. The heat treatment used to inactivate virus samples for safety testing may have damaged some viral proteins, potentially explaining the few false negative results. Testing with fresh samples would provide more accurate performance metrics.
Cost considerations extend beyond materials to include antibody production and storage requirements. Although the sensor components cost only 20 cents, the specific antibodies required represent a more significant expense and need refrigeration, which could complicate distribution in resource-limited settings.
The device's adaptability to other pathogens depends on antibody availability and stability when attached to the magnetic nanoparticles. Some antibodies may not maintain their binding specificity after chemical attachment, requiring optimization for each new target.
Despite these challenges, the core innovation demonstrates remarkable potential. The integration of waste recovery, 3D printing, and magnetic separation creates a replicable platform that could transform point-of-care diagnostics globally.
Looking Forward
This recycled biosensor represents more than just a COVID-19 test; it's a proof-of-concept for sustainable, accessible healthcare technology. The platform's modular design enables rapid adaptation to new pathogens by simply changing capture antibodies, making it valuable for pandemic preparedness and routine surveillance.
The economic and environmental benefits extend beyond individual tests. If adopted at scale, such technologies could significantly reduce healthcare waste while improving diagnostic access in underserved communities. The approach also provides a model for other medical devices, suggesting how circular economy principles might transform healthcare manufacturing.
For researchers and healthcare developers, this work provides detailed protocols for recycled sensor fabrication, magnetic bioconjugate preparation, and clinical validation methods. These resources could accelerate similar innovations addressing other global health challenges while promoting environmental sustainability.
Definitions
Biosensor: A device that uses biological components (like antibodies) to detect specific molecules, converting the detection event into a measurable signal.
Electrochemical detection: A measurement technique that monitors changes in electrical current or voltage to identify the presence and quantity of target molecules.
Magnetic nanoparticles: Extremely small particles (nanometer scale) that respond to magnetic fields, used here to capture and concentrate viral proteins for detection.
Superparamagnetic: A magnetic property where particles become strongly magnetized in a magnetic field but lose magnetization when the field is removed, preventing clumping.
Antibody conjugation: The chemical process of attaching antibodies to nanoparticles so they can specifically bind to target proteins while being magnetically recoverable.
Spike protein (S1-RBD): The part of the SARS-CoV-2 virus that binds to human cells, serving as a key target for detection and vaccines.
Limit of detection (LOD): The smallest amount of a substance that can be reliably detected by an analytical method.
Sensitivity and specificity: Measures of test accuracy; sensitivity indicates the percentage of actual positives correctly identified, while specificity indicates the percentage of actual negatives correctly identified.
References: (Carvalho et al., 2025); (Crapnell and Banks, 2023); (Kalinke et al., 2024); (Huang et al., 2022); (Chaibun et al., 2021).


Sustainable Electrochemical-Magnetic Biosensor From Recycled Materials
Sustainable Electrochemical-Magnetic Biosensor Fabricated from Recycled Materials for Label-Free Detection of SARS-CoV-2 in Human Saliva