Achieving the precise construction of chiral molecules is essential for progress in pharmaceuticals, materials science, and biochemistry. Recent research has introduced a groundbreaking approach: photoinduced copper catalysis for the deracemization of alkyl halides, marking a significant advance in asymmetric synthesis and the creation of enantioenriched compounds.
Key Advances Transforming the Field
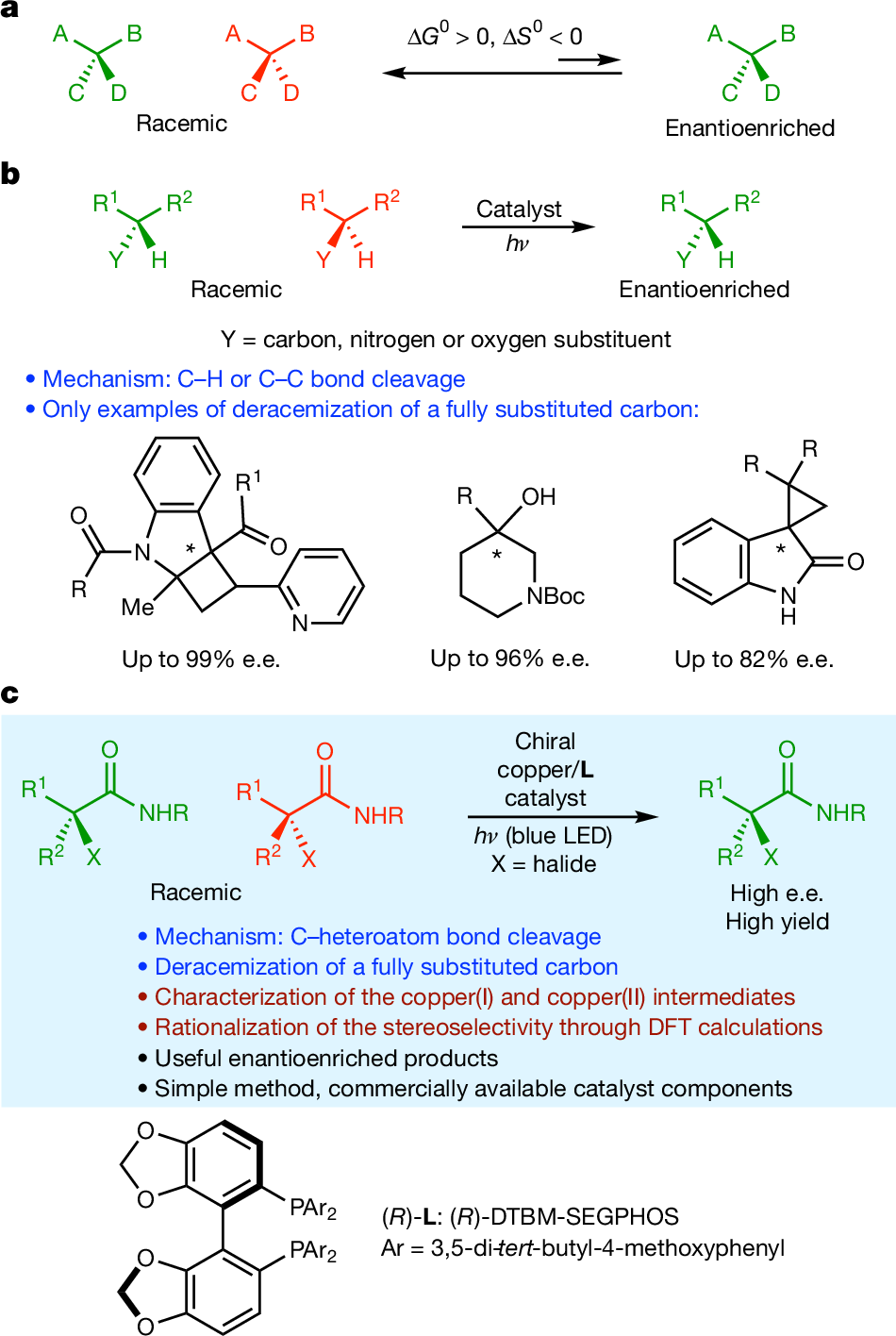
- Innovative Mechanism: The method pioneers a deracemization route via carbon-halogen bond cleavage, surpassing traditional carbon-hydrogen and carbon-carbon cleavage strategies.
- Enhanced Access to Stereocenters: It enables efficient deracemization of tertiary and secondary alkyl halides, even those with tetrasubstituted stereocenters, delivering high yields and outstanding enantiomeric excess.
- Practical and Scalable: The chiral copper catalyst is easily generated in situ from CuCl and DTBM-SEGPHOS, exhibits excellent tolerance to air and water, and is scalable to gram quantities without compromising performance.
- Broad Applicability: This approach distinguishes among primary, secondary, and tertiary alkyl groups and works with various α,α-dialkyl-α-haloamides and similar substrates.
- Mechanistic Insight: Employing photophysical studies and DFT calculations, the researchers clarified the origins of enantioselectivity, guiding future catalyst development.
- Synthetic Versatility: The resulting chiral alkyl halides serve as intermediates for creating C-F, C-C, C-N, and C-O bonds, and are amenable to streamlined, one-pot syntheses.
Overcoming Traditional Barriers in Deracemization
Deracemization transforms a racemic mixture into a single enantiomer, offering unmatched potential for optimizing both bond formation and stereocontrol. Historically, this process faced significant thermodynamic and kinetic obstacles, as racemates are more stable and thermal reversals are common.
Photochemistry solves these issues by leveraging excited states to bypass conventional barriers. Until now, photoinduced deracemization was mostly limited to specific structures and rarely effective for tetrasubstituted stereocenters.
The new strategy uses a chiral copper catalyst to achieve photoinduced deracemization via carbon-halogen bond cleavage, greatly expanding the method’s reach. This opens new possibilities for synthesizing complex molecules, particularly those with tetrasubstituted stereocenters, key motifs in many pharmaceuticals and bioactive compounds.
Implications for Industry and Research
This breakthrough promises wide-ranging benefits:
- Versatile Carbon-Halogen Bond Cleavage: Expands the applicability of deracemization to diverse synthetic targets in drug development and materials science.
- Mastery of Stereocenters: Efficiently handles tetrasubstituted stereocenters, a crucial challenge in asymmetric synthesis, with direct relevance to bioactive molecules.
- Industrial Readiness: The method’s robustness, air/water tolerance, readily available reagents, and scalability, makes it attractive for commercial applications.
- Flexible Intermediates: Chiral alkyl halides enable the synthesis of a broad spectrum of valuable, enantioenriched compounds.
Experimental Insights and Mechanism
The procedure utilizes CuCl, DTBM-SEGPHOS, and blue LED irradiation to achieve high yields and enantioselectivities, even at gram scale. Both the copper catalyst and light are essential for success. The methodology tolerates numerous functional groups, though some amines and quinolines may impede the reaction by binding copper.
The general procedure for deracemization of α,α-dialkyl-α-chloroamides involves stirring CuCl (10 mol%) and (R)-DTBM-SEGPHOS (12 mol%) in anhydrous 2-methyltetrahydrofuran (2-Me-THF) at room temperature, followed by the addition of the racemic electrophile solution. The reaction mixture is then cooled to -78 °C and irradiated with blue LEDs for 48 hours. Similar procedures are outlined for α,α-dialkyl-α-bromoamides, α-alkyl-α-aryl-α-chloroamides, and α-aryl-α-chloroamides, with minor adjustments to the copper source, solvent, or reaction time.
Mechanistic analysis, supported by NMR, EPR spectroscopy, and DFT, reveals a catalytic cycle that begins with copper(I) photoexcitation, followed by electron transfer to form a copper(II) complex and an organic radical. The key halogen-atom transfer step ultimately determines enantioselectivity, with DFT calculations highlighting how subtle steric and electronic effects favor the desired enantiomer, an outcome confirmed by experimental data.
Takeaway: A Blueprint for Future Innovation
This research sets a new precedent for chiral synthesis. By harnessing photoinduced copper-catalyzed deracemization through carbon-halogen bond cleavage, scientists have unlocked a powerful, efficient, and practical method for constructing chiral molecules. Their detailed mechanistic understanding paves the way for further breakthroughs, ensuring this strategy's central role in advancing pharmaceuticals, agrochemicals, and advanced materials.

Photoinduced Copper Catalysis For Chiral Synthesis
Photoinduced copper-catalysed deracemization of alkyl halides