A new bioRxiv preprint from Weill Cornell Medicine and collaborators reports a structure-guided, AI-assisted campaign to discover and optimize small-molecule agonists of Triggering Receptor Expressed on Myeloid Cells 2 (TREM2), a microglial receptor implicated in Alzheimer's disease (AD) risk and microglial protective programming.
The team screened more than five million purchasable compounds with a Deep Docking workflow, validated hits biophysically, advanced structure–activity relationships (SAR) by catalog, and demonstrated functional cellular activity consistent with TREM2 agonism.
The study identifies T2M-010 as a submicromolar TREM2 binder (Kd 0.83 ± 0.10 μM) that triggers proximal signaling (SYK phosphorylation) and enhances microglial phagocytosis, while showing favorable in vitro ADME/PK properties aligned with central nervous system (CNS) exposure.
The authors conclude that T2M-010 is, to their knowledge, the most potent small-molecule TREM2 binder reported to date, and that their pipeline offers a roadmap for oral, brain-penetrant immunomodulators in AD pathology.
Background
Alzheimer's disease (AD) features amyloid-β plaques, tau tangles, and chronic neuroinflammation, with microglia, the brain's resident immune cells, shaping tissue responses.
Genetic and mechanistic studies implicate the microglial receptor TREM2 in these responses: loss-of-function variants increase AD risk and blunt microglial recruitment, plaque compaction, and protective programming, while increased TREM2 activity supports debris clearance and neuronal protection (Deczkowska et al., 2020); (Ulland and Colonna, 2018).
Antibody agonists of TREM2 have demonstrated microglial activation and plaque remodeling in models, but translation can be hindered by limited blood–brain barrier (BBB) penetration, manufacturing cost, and potential immunogenicity.
Small molecules, by contrast, can be optimized for oral dosing, BBB penetration, and tunable pharmacokinetics, making them attractive complements or alternatives for neuroimmune modulation (Cho et al., 2025).
Key Takeaways
- Deep Docking of ~5M compounds surfaced a novel hit (T2K-014), leading to an optimized analog (T2M-010) with Kd 0.83 μM for TREM2.
- T2M-010 induced SYK phosphorylation in TREM2-expressing cells and increased microglial phagocytosis, consistent with functional agonism.
- In vitro PK suggests CNS suitability with high passive BBB permeability (PAMPA-BBB), robust MDCK–MDR1 A→B transport with low efflux, moderate logD7.4, and acceptable microsomal stability.
- Safety counterscreens showed no cytotoxicity in human fibroblasts up to 100 μM and low hERG inhibition risk at tested ranges.
- Indole at R1 appears essential for binding in this series; R2 tolerates diversity and offers tunability.
- Findings strengthen the therapeutic rationale for small-molecule TREM2 agonists as complements or alternatives to antibodies.
Study Overview
The team used AI-assisted virtual screening (Deep Docking) to triage ~5 million purchasable compounds against a TREM2 pocket spanning hydrophobic and basic residues informed by the natural product Hecubine (Li et al., 2024). From the top 30 virtual hits purchased, one molecule, T2K-014, showed measurable binding to recombinant human TREM2 and became the seed for rapid SAR exploration.
Methods In Brief
Deep Docking (virtual screening) is a machine-learning approach that predicts docking scores to triage very large libraries, enabling explicit docking and experimental follow-up of the most promising candidates. Here, ~5 million purchasable compounds were prioritized against a TREM2 pocket spanning hydrophobic and basic residues informed by Hecubine (Li, 2024).
TRIC (Dianthus): biophysical hit confirmation via temperature-related fluorescence intensity change to detect protein–ligand binding.
MST: microscale thermophoresis to quantify dissociation constants (Kd).
AlphaLISA: proximity assay to quantify SYK phosphorylation as a downstream readout of TREM2 activation.
PAMPA-BBB: in vitro passive permeability across a BBB-like membrane;
MDCK–MDR1: cell-based transport and efflux to estimate P-gp interaction.
Why It Matters
Microglia orchestrate the innate immune response in the brain. In AD, chronic neuroinflammation and impaired debris clearance correlate with neuronal injury and disease progression. Genetic and functional studies implicate TREM2 signaling in the transition of microglia toward a protective, disease-associated state that compacts plaques and reduces neuritic damage. Therefore, pharmacologically boosting TREM2 activity is a compelling strategy to modulate microglia toward beneficial results.
Small-molecule TREM2 agonists could complement antibody approaches by offering brain penetration, oral convenience, and flexible dose titration. If efficacy and safety translate in vivo, such agents could integrate with existing AD therapies or future combination regimens focused on neuroimmune modulation.
Discussion
Hit Discovery and Binding Mode: The Deep Docking campaign yielded T2K-014, a chemotype with low structural similarity to Hecubine and a distinct interaction profile. Docking suggested primary contacts with hydrophobic site residues (W44, L71, L89), in contrast to Hecubine's engagement of basic site residues (R76, T85, D87) and F74. This divergence underscores the benefit of exploring large chemical space with ML-prioritized docking to identify novel binding solutions.
Figure 1 depicts the binding site partitions, the screening workflow, and the predicted T2K-014 pose; together they contextualize how the authors bridged in silico triage with wet-lab traction.
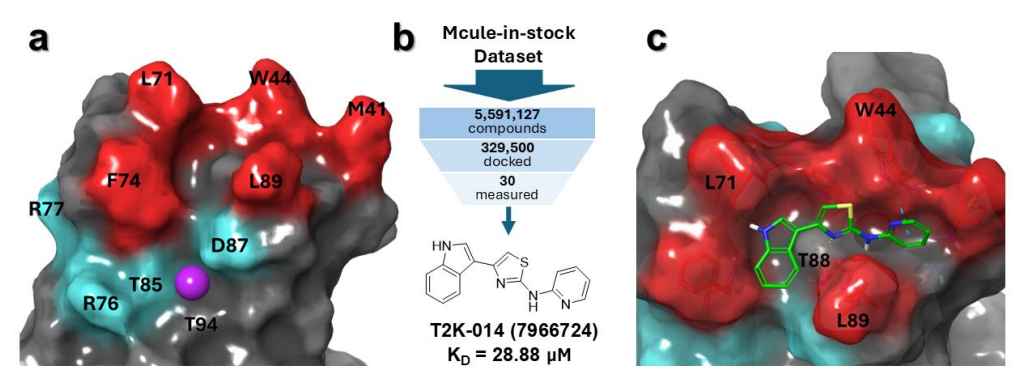
Figure 1. Discovery of TREM2-binding compounds via virtual screening. (a) TREM2 binding sites used for virtual screening. Red/cyan residues correspond to the hydrophobic and basic sites, respectively.24 The purple sphere represents the proposed binding site of Hecubine.21 (b) Overview of the virtual screening workflow. With Deep Docking, computational efficiency is maximized by training a deep learning model on a minority of the dataset that is actually docked. (c) Predicted binding mode of the confirmed hit T2K-014, with the interacting residues labeled. Protein-ligand interactions are shown as dashed lines, with yellow and light blue coloring meaning hydrogen bonds, and pi-pi interactions, respectively. Credit: Gabr et al.
Biophysical Validation: Using TRIC on the Dianthus platform, the authors screened 30 candidates at 100 μM against 10 nM recombinant TREM2. Only T2K-014 produced a binding signal near the predetermined hit threshold; Microscale Thermophoresis (MST) quantified Kd at 28.88 ± 1.74 μM. Figure 2A visualizes single-point TRIC calls with controls and hit criteria while Figure 2B shows the dose response curve used to derive affinity. Although modest, this affinity justified SAR-by-catalog exploration.
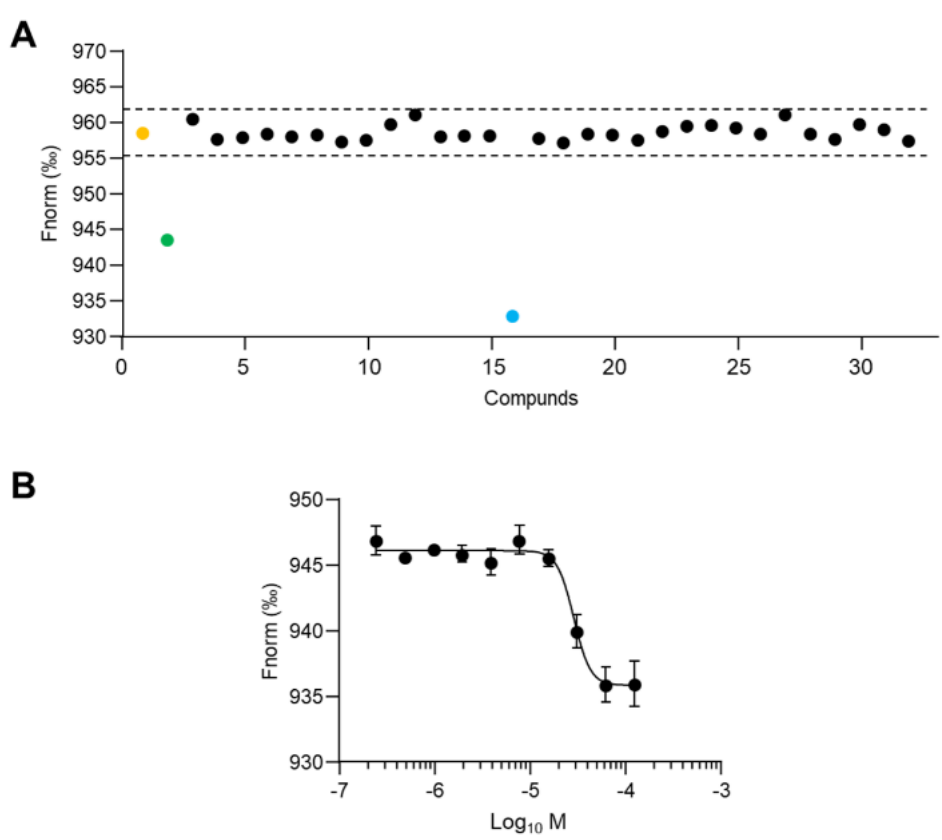
Figure 2. Characterization of selected virtual screening hits for TREM2 binding. A) Biophysical validation of 30 computationally selected compounds using TRIC analysis. Compounds were tested at 100 μM against recombinant TREM2 protein (10 nM). Yellow dot: baseline control (TREM2 alone); green dot: reference compound PC-192. Dashed lines denote hit selection criteria based on 5-fold standard deviation above background signal. Blue dot represents the confirmed binding compound (T2K-014); black dots indicate non-binding compounds. B) Dose-response curve using MST for the binding of T2K-014 to TREM2. Data represent mean ± SEM (n = 3). Credit: Gabr et al.
SAR and Improved Affinity: Twenty-seven analogs probed R1 (indole region) and R2 (pyridine region) substitutions. Four derivatives exceeded binding thresholds in TRIC and advanced to MST: T2M-002 (Kd 50.84 ± 3.74 μM), T2M-003 (1.35 ± 0.16 μM), T2M-016 (2.44 ± 0.30 μM), and T2M-010 (0.83 ± 0.10 μM).
Three analogs substantially outperformed the parent, with T2M-010 exhibiting the strongest binding (~35-fold better than T2K-014). Figure 3 captures the screening cascade, structures of active analogs, and their concentration-dependent profiles. A recurring motif across actives was the preservation of the indole at R1, suggesting an essential pharmacophore, while R2 tolerated more diversity, offering a handle for tuning potency and selectivity.
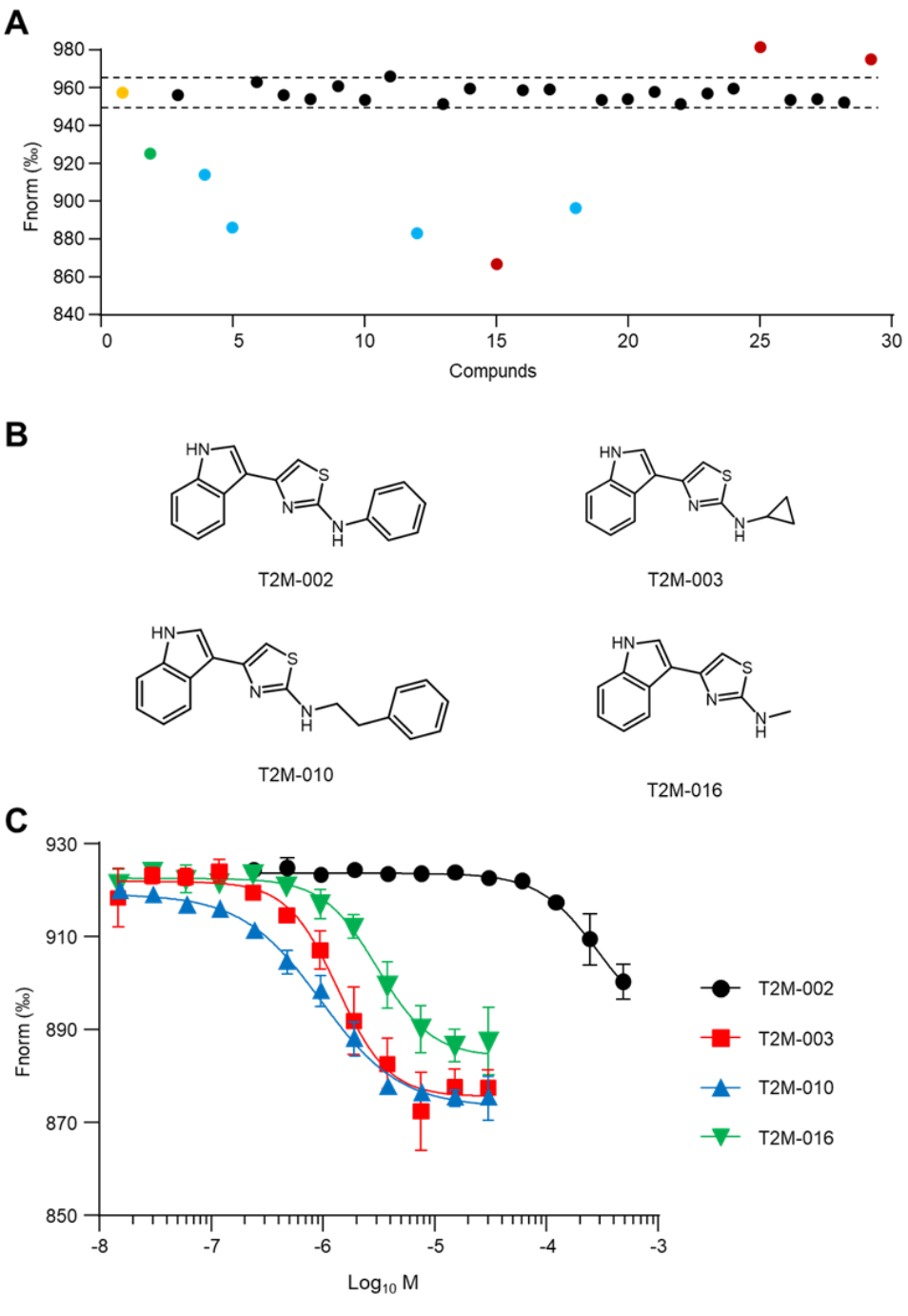
Figure 3. SAR-by-catalog study of T2K-014 derivatives. A) Single-concentration screening of 27 T2K-014 derivatives against TREM2 protein (10 nM) at 100 μM using TRIC analysis. Yellow dot: negative control (TREM2 alone); green dot: positive control (T2K-014). Dashed lines represent the threshold for hit identification, corresponding to signals exceeding 5-fold the standard deviation of the negative control. Blue dots indicate validated TREM2- binding hits; red dots represent compounds causing aggregation or fluorescence interference. B) Chemical structures of four T2K-014 derivatives identified through TRIC screening: T2M-002, T2M003, T2M-010, and T2M-016. C) Concentration-dependent binding profiles of T2K-014 derivatives determined by MST. Black circles: T2M-002; red squares: T2M-003; blue triangles: T2M-010; green inverted triangles: T2M-016. Data are presented as mean ± SEM (n = 3). Credit: Gabr et al.
Functional Agonism: To test whether tighter binding translated to signaling, the team measured TREM2-proximal SYK phosphorylation in HEK cells co-expressing hTREM2/DAP12. At 25 μM (1 h), T2M-010 roughly doubled phospho-SYK levels relative to vehicle, mirroring the effect of the benchmark small-molecule agonist VG-3927; T2M-016 produced only a modest, non-significant increase.
Figure 4 summarizes the AlphaLISA results, supporting the conclusion that T2M-010 engages TREM2 in a productive, agonistic orientation rather than acting as a non-functional binder.
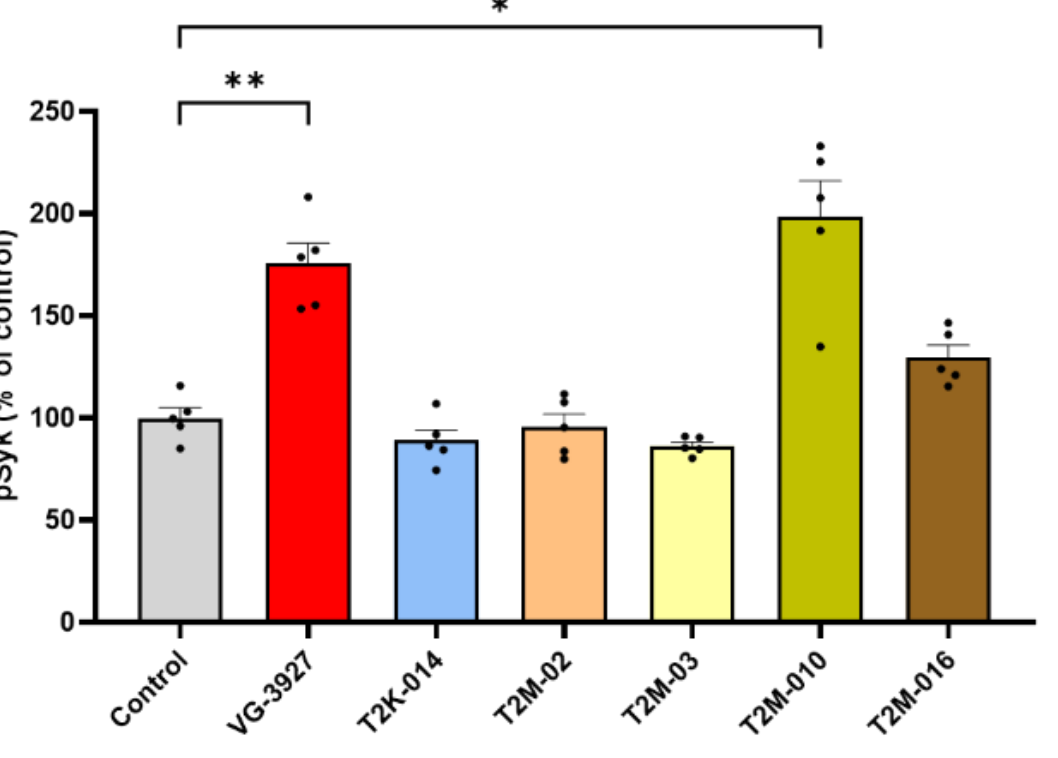
Figure 4. Relative changes in SYK phosphorylation in response to TREM2 agonists, measured by AlphaLISA. Histogram illustrating the relative levels of phosphorylated SYK (Tyr525/526) in HEK–hTREM2/DAP12 cells under control conditions and following treatment with candidate TREM2 agonists: T2K-014, T2M-02, T2M-03, T2M-010 and T2M-016 at 25 µM for 1 hour. Additionally, cells were treated with the commercial TREM2 agonist VG3927 (25 µM, 1 h) as a reference compound. Phospho-SYK levels were quantified using the AlphaLISA SureFire Ultra assay and are expressed as a percentage relative to the control condition (vehicletreated cells). Data represent the mean ± SEM from five independent biological replicates. Statistical significance was assessed using Brown-Forsythe and Welch Anova test followed by Dunnett ’s multiple comparison test (*p <0.05, **p < 0.01). Credit: Gabr et al.
Microglial Effector Function: In BV2 microglia, T2M-010 significantly enhanced phagocytic uptake of fluorescent beads, again comparable to VG-3927. Figure 5 quantifies the percent of IBA1-positive microglia containing at least one internalized bead across replicates, with stringent statistics (Brown-Forsythe and Welch ANOVA with Dunnett's test; ****
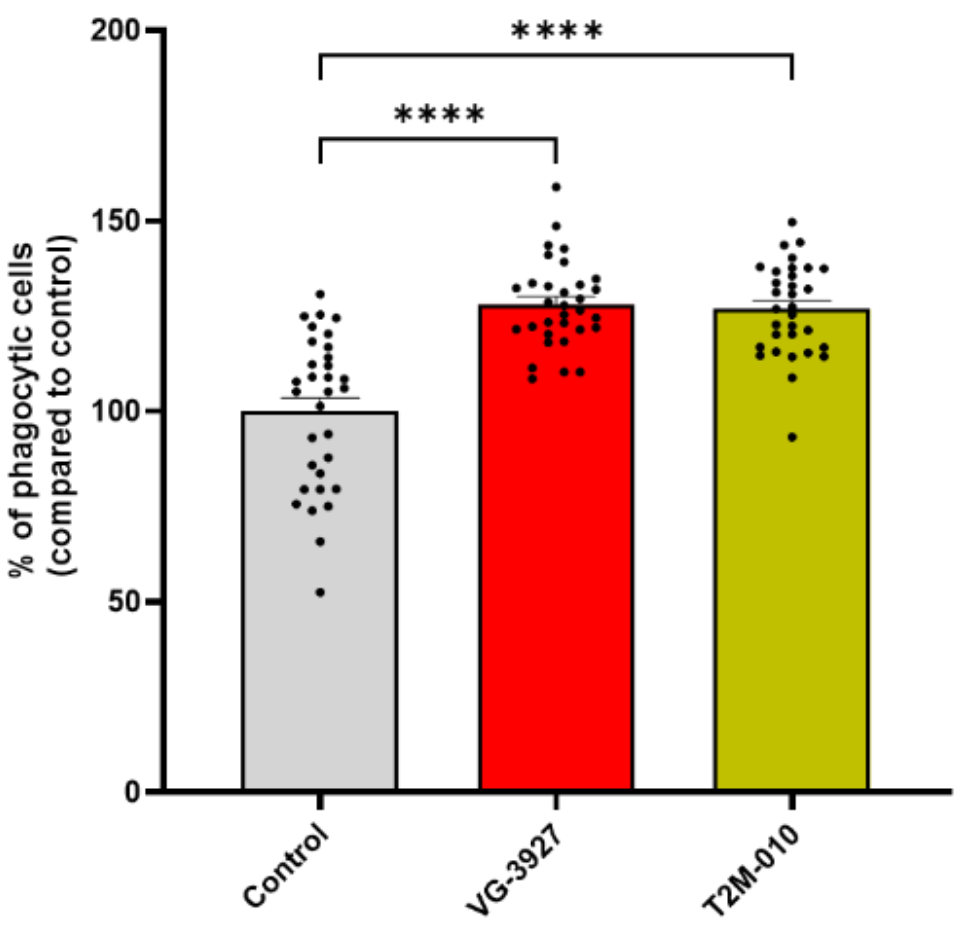
Figure 5. Quantification of phagocytic activity in BV2 microglial cells. BV2 cells were pre-treated with the indicated compounds (T2M-010 or VG-3927) at a concentration of 25 µM, or vehicle control (DMSO) for 30 minutes, followed by exposure to green fluorescent latex beads. Phagocytic cells were defined as IBA1-positive cells containing at least one internalized fluorescent bead. Quantification is expressed as the percentage of phagocytic cells relative to vehicle control. Data represent mean ± SEM from 32 images per group across four independent biological replicates (n = 4). Statistical significance was assessed using Brown-Forsythe and Welch ANOVA followed by Dunnett’s multiple comparisons test. ****p < 0.0001. Credit: Gabr et al.
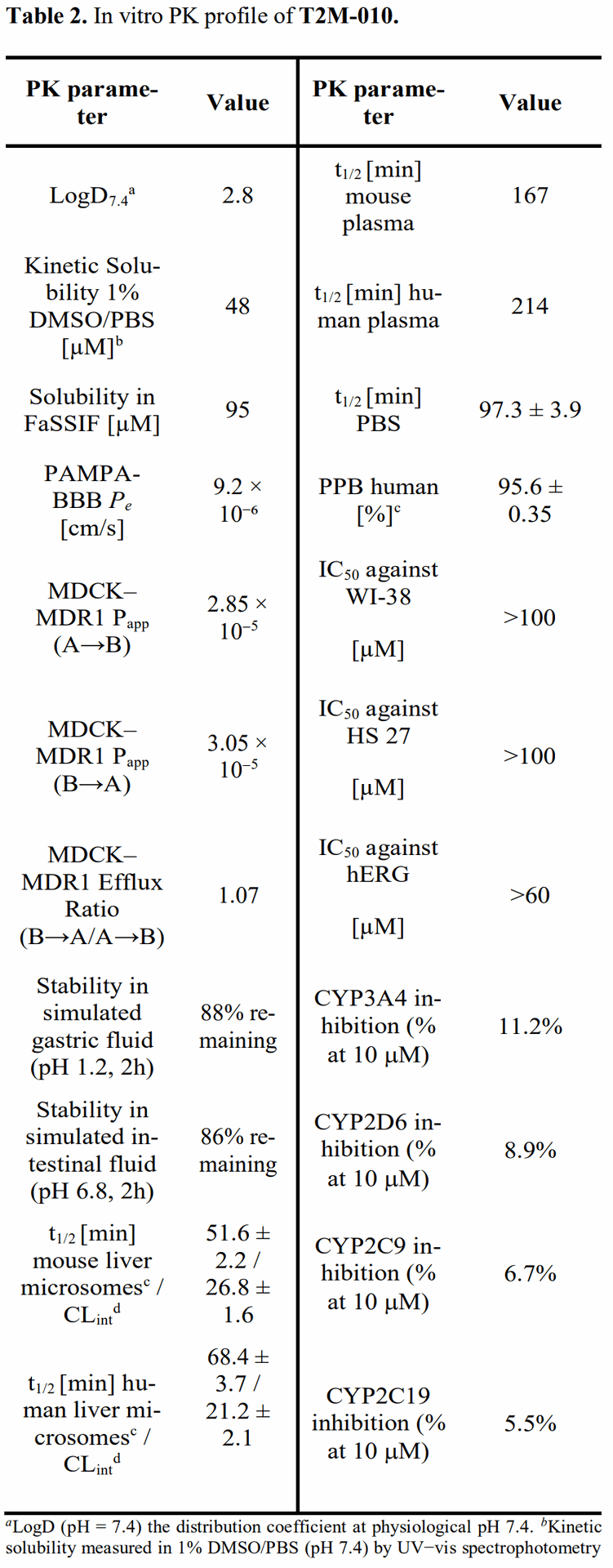
In Vitro ADME/PK and Safety: T2M-010 displayed properties aligned with CNS exposure and developability. Highlights include moderate logD7.4 (2.8), good kinetic and biorelevant solubility (PBS and FaSSIF), high passive permeability in PAMPA-BBB (Pe ~ 9.2 × 10^−6 cm/s), robust MDCK–MDR1 transport with low efflux (ER ~ 1.07), long plasma half-life (mouse 167 min; human 214 min), moderate microsomal stability with acceptable projected clearance, high plasma protein binding (PPB ~ 96%), and clean counterscreens in human fibroblasts (IC50 > 100 μM) and hERG (> 60 μM at tested conditions). Table 2 in the paper collates these metrics and their assay conditions.
Limitations and Next Steps
As a preprint, the results await peer review, and the data are predominantly in vitro with cellular function readouts. Key next steps include in vivo pharmacokinetics, brain exposure and receptor engagement, microglial state profiling in disease-relevant models, dose–response relationships for efficacy, safety pharmacology beyond hERG and cytotoxicity, and medicinal chemistry to balance potency, selectivity, and pharmacokinetics. Additionally, orthogonal binding assays and structural studies (e.g., cryo-EM, NMR, mutagenesis) could illuminate binding determinants and support rational design at R2.
Conclusion
This work demonstrates a credible path from multi-million compound AI triage to a submicromolar, functionally active small-molecule agonist of TREM2 with a brain-friendly in vitro PK profile.
The optimized analog T2M-010 reinforces the therapeutic case for microglial modulation in AD and expands chemical diversity beyond prior modalities. If future in vivo studies confirm brain penetration, target engagement, and disease-modifying effects with an acceptable safety window, T2M-010-like chemotypes could become compelling candidates for clinical development, either alone or in combination with other neuroimmune strategies.
Importantly, the authors position the series as a starting point rather than an end state. The emerging SAR suggests room to tune potency and selectivity at R2 while preserving the indole pharmacophore at R1. Combining iterative design with mechanistic assays and in vivo validation will determine whether small-molecule TREM2 agonism can deliver on its promise in human disease.
Call to Action
What do you think about small-molecule immunomodulation for AD? Share your thoughts, or read the preprint and join the discussion on how to translate TREM2 agonism into the clinic.
Definitions
TREM2: Triggering Receptor Expressed on Myeloid Cells 2; a microglia-enriched surface receptor that signals via DAP12/TYROBP to regulate phagocytosis, survival, and activation states.
Deep Docking: A machine learning-augmented virtual screening approach that predicts docking scores to prioritize compounds for explicit docking and experimental testing across very large libraries.
TRIC (Dianthus): Temperature-Related Intensity Change; a biophysical method to monitor protein–ligand interactions by fluorescence intensity changes as a function of temperature.
MST: Microscale Thermophoresis; quantifies binding affinities by measuring molecule movement along temperature gradients.
Microglia: resident immune cells of the central nervous system that survey the brain, clear debris, and orchestrate inflammatory responses.
DAP12/TYROBP and ITAM: an adaptor protein (TYROBP) containing an immunoreceptor tyrosine-based activation motif (ITAM) that couples TREM2 to intracellular kinases such as SYK.
SYK phosphorylation: activation of spleen tyrosine kinase, a proximal signal downstream of ITAM-coupled receptors, used here as a readout of TREM2 signaling.
BBB: the blood–brain barrier, a selective interface that limits compound entry into the brain.
ADME/PK: absorption, distribution, metabolism, excretion, and pharmacokinetics—properties that determine exposure and dosing.
PAMPA-BBB, MDCK–MDR1, P-gp: in vitro assays estimating passive BBB permeability (PAMPA-BBB) and transporter-mediated efflux across a cell monolayer (MDCK–MDR1), often mediated by P-glycoprotein (P-gp).
References
Primary paper: (Cho et al., 2025) AI-Assisted Discovery and Optimization of Small Molecule TREM2 Agonists with Functional Microglial Activity. bioRxiv.
Background on TREM2 signaling and microglia: (Deczkowska et al., 2020) The Physiology, Pathology, and Potential Therapeutic Applications of the TREM2 Signaling Pathway. Cell.
Microglial biology in AD: (Ulland and Colonna, 2018) TREM2 - a key player in microglial biology and Alzheimer disease. Nature Reviews Neurology.
Natural product activator of TREM2 used to define binding site: (Li et al., 2024) Hecubine as a New TREM2 Activator. Redox Biology.
Deep Docking methodology: (Gentile et al., 2020) Deep Docking: A Deep Learning Platform for Augmentation of Structure-Based Drug Discovery. ACS Central Science.

AI-Assisted Discovery of Small-Molecule TREM2 Agonists That Activate Microglia
AI-Assisted Discovery and Optimization of Small Molecule TREM2 Agonists with Functional Microglial Activity