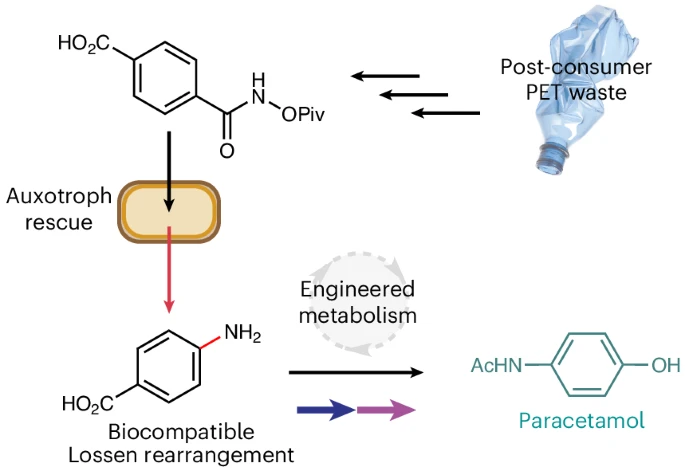
In a landmark study published in Nature Chemistry (Johnson et al., 2025), researchers from the University of Edinburgh and AstraZeneca have demonstrated a biocompatible version of the Lossen rearrangement inside living Escherichia coli cells.
This innovation enables the conversion of plastic waste into valuable amine-containing metabolites, including the over-the-counter drug paracetamol, by merging synthetic chemistry with microbial metabolism. The work, led by Nick W. Johnson represents a significant advance in sustainable chemical manufacturing and bioremediation.
Credit: Nature
Key Takeaways
- First demonstration of a non-enzymatic Lossen rearrangement operating inside living E. coli cells.
- The reaction is catalyzed by phosphate, is non-toxic, and proceeds under mild, biocompatible conditions.
- Researchers engineered E. coli to depend on the Lossen rearrangement for growth, using it to generate the essential metabolite para-aminobenzoic acid (PABA).
- The Lossen substrate can be synthesized from polyethylene terephthalate (PET) plastic waste, enabling upcycling of post-consumer plastics.
- Integration with engineered metabolic pathways allows the production of paracetamol (acetaminophen) from PET-derived feedstocks.
- This approach expands the synthetic capabilities of living cells, bridging the gap between chemical and biological manufacturing.
Expanding the Chemistry of Life: An Overview
Nature’s metabolic toolkit is powerful but limited. Synthetic organic chemistry, by contrast, offers a vast array of reactions not found in biology. The Lossen rearrangement, discovered in 1872, transforms acyl hydroxamates into primary amines via a migration and decarboxylation process. Traditionally, this reaction requires harsh or non-biological conditions. The authors sought to bridge this gap by developing a version compatible with living cells.
The team focused on E. coli, a workhorse of biotechnology, and designed a system where the Lossen rearrangement would generate PABA, an essential precursor for folic acid biosynthesis.
By using a PABA-auxotrophic strain (unable to grow without external PABA), they could directly link the success of the reaction to cell growth. Remarkably, they found that the reaction proceeded efficiently in standard growth media, catalyzed by phosphate ions, and did not require any toxic metals or engineered enzymes.
This biocompatible chemistry was then coupled to the upcycling of PET plastic. The researchers synthesized the Lossen substrate from terephthalic acid, a monomer obtained by hydrolyzing PET bottles. When fed to the engineered E. coli, this substrate was converted to PABA, supporting cell growth and opening a path from plastic waste to valuable metabolites.
Why This Matters: Sustainability and Synthetic Biology
The integration of abiotic (non-biological) chemical reactions into living cells is a frontier in synthetic biology and green chemistry. Traditional biomanufacturing is limited by the reactions that enzymes can catalyze. By introducing new-to-nature reactions like the Lossen rearrangement, scientists can expand the range of chemicals that microbes can produce from renewable or waste feedstocks.
This work is particularly significant for plastic waste remediation. PET is one of the most widely used plastics, with over millions of tons of waste generated annually. Current recycling methods are energy-intensive and often downcycle the material. The approach described here not only breaks down PET but also transforms it into high-value products, such as pharmaceuticals, using engineered bacteria.
Moreover, the study demonstrates that such reactions can be harnessed to control microbial growth and metabolism, offering new strategies for metabolic engineering, bioproduction, and even synthetic ecosystem design.
Results and Figures: From Reaction Discovery to Drug Synthesis
The authors’ experimental journey began with an auxotroph rescue assay (see Figure 2 in the original paper), where the growth of PABA-deficient E. coli was restored by the Lossen rearrangement of a synthetic substrate.
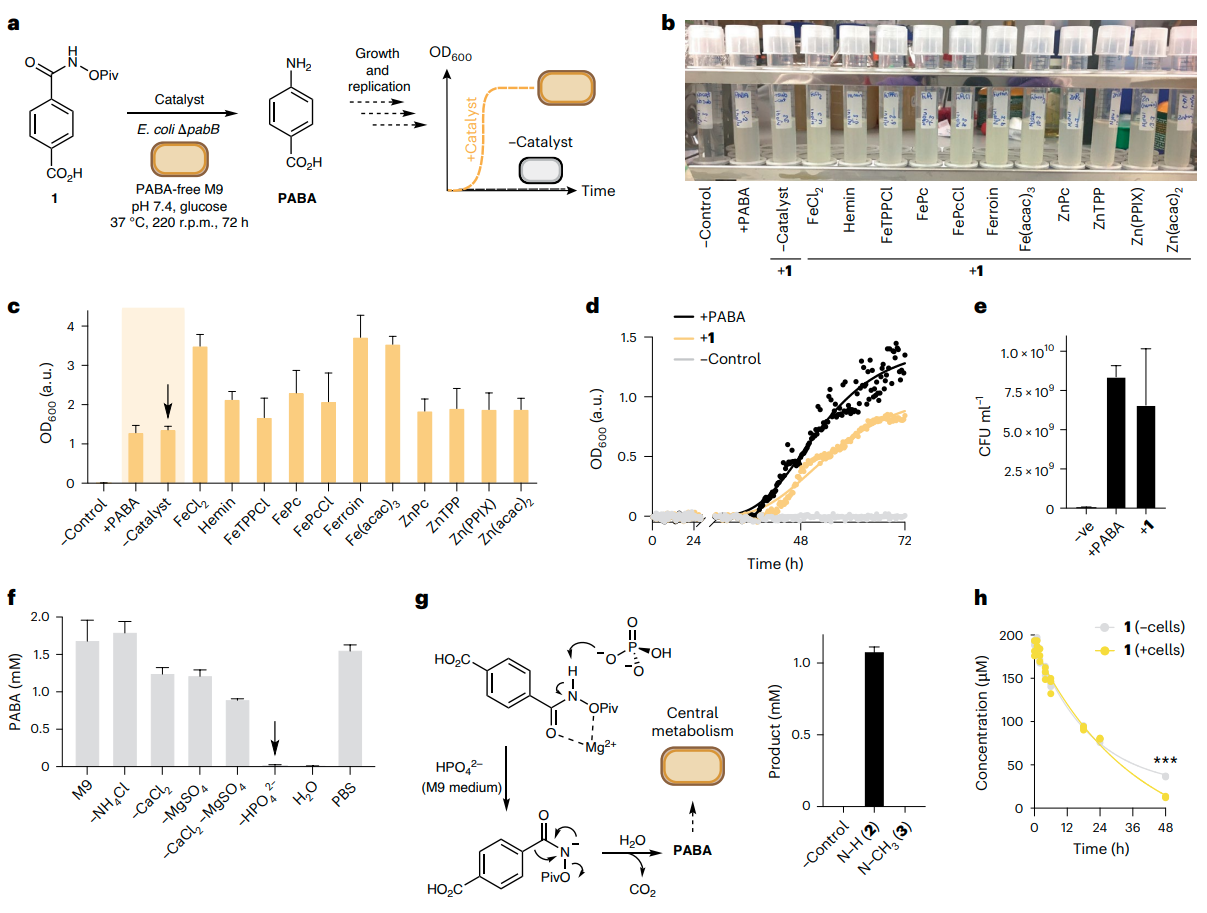
Fig. 2 | Reaction discovery by auxotroph rescue. a, Auxotroph rescue experiment via Lossen rearrangement. b, A photograph of cultures after the auxotroph recovery of E. coli BW25113∆pabB in the presence of 1 and various transition-metal catalysts. c, Culture density measurements by spectrophotometry. d, Auxotroph rescue growth curves from cultures of E. coli BW25113∆pabB grown in the presence of PABA or 1. The extended lag phase is due to the 105 -fold dilution of the starting inoculum that is required to ensure no background growth without PABA. e, Plate-count assay from cultures of E. coli BW25113∆pabB grown in the presence of PABA or 1. f, In vitro experiment examining the effect of M9 media components on the Lossen rearrangement of 1. g, The proposed phosphate-catalysed Lossen rearrangement of 1 to PABA and in vitro reactivity of O-acylated and N-methylated control compounds 2 and 3. h, Substrate depletion assay using 200 µM 1 in the presence and absence of metabolically active cells. ***P < 0.0005 (t-test). All data are presented as mean values ± s.d. of three biological replicates. Credit: Nature
They systematically ruled out enzymatic catalysis, showing that phosphate ions in the medium were responsible for the reaction. The substrate was non-toxic and effective across a range of concentrations.
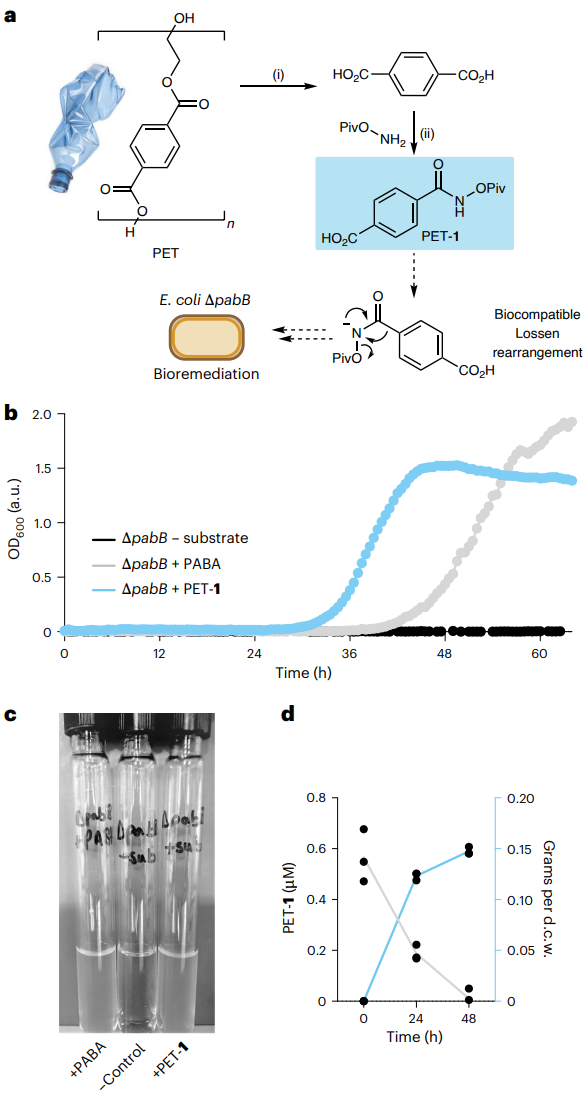
Fig. 3 | Substrate synthesis from PET plastic waste for bioremediation.
a, Synthesis of the Lossen rearrangement substrate from PET waste and PET
bioremediation strategy via auxotroph recovery. b, Growth curves from 96-well
plate experiments during auxotroph rescue. Error bars are omitted for clarity.
c, A photograph of auxotroph rescue experiments using a PET-derived substrate.
d, Substrate depletion (grey) and dry cell weight (d.c.w.) production (blue)
during growth experiments in Falcon tubes using 1 µM PET-1. A conversion factor
of 0.33 grams per litre per OD600 was applied. All data are shown as triplicate
experiments to one standard deviation. (i) NaOH, EtOH/H2O, reflux, 10 h. (ii) T3P,
iPr2NEt, H2NOPiv·TfOH, tetrahydrofuran, 0 °C to room temperature, 16 h. Credit: Nature
Next, the team synthesized the Lossen substrate from PET waste and demonstrated that it could support bacterial growth as efficiently as pure PABA (Figure 3). This established a direct link between plastic waste and microbial metabolism.
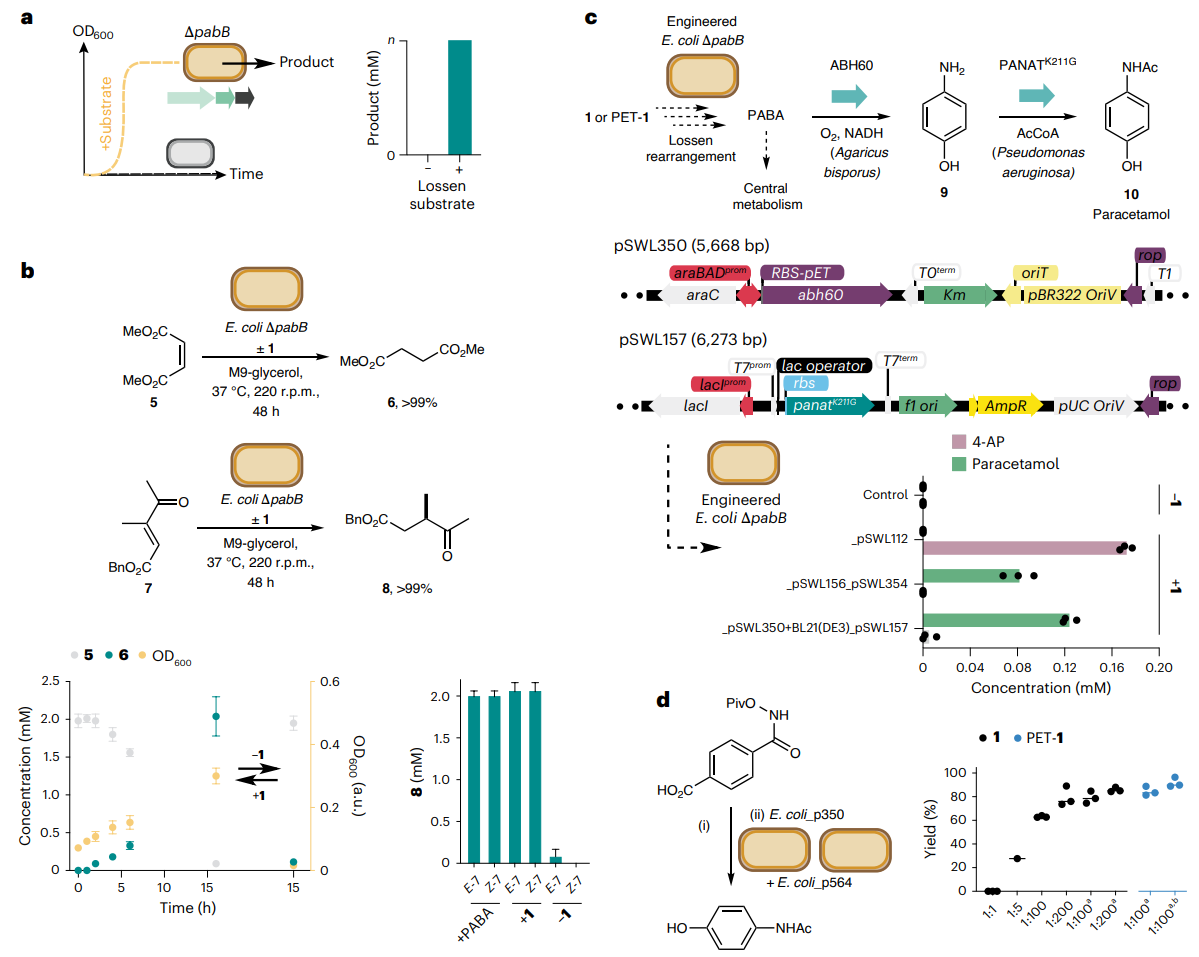
Fig. 4 | Interfacing the Lossen rearrangement with native and engineered
metabolic pathways. a, Interfacing the Lossen rearrangement with native
and engineered biosynthetic pathways in E. coli. b, Biotransformation of DMM
(5) to dimethylsuccinate (6) and β-ketoacrylate 7 to γ-ketoester 8 using E. coli
BW25113∆pabB and 1 or PET-1. c, A de novo biosynthetic pathway to 4-AP (9) and
paracetamol 10 incorporating a non-enzymatic Lossen rearrangement, plasmid
designs and whole-cell production experiments. d, Paracetamol synthesis by
one-pot Lossen rearrangement and bacterial whole-cell synthesis. Strain
E. coli_p350 expresses ABH60 and strain E. coli_p354 expresses PANATK211G.
Ratios refer to E. coli_p354 and E. coli_p350 in biotransformations (1:1, OD600 26;
1:5, OD600 16; 1:100 and 1:200, OD600 12.5). a
Final cell density OD600 25. OD refers
to the final optical density at 600 nm. b
Cells induced with 0.5% l-arabinose.
Reaction conditions: (i) substrate (0.5 mM), aqueous potassium phosphate
(200 mM, pH 8.0), 50 °C, 48 h; (ii) E. coli_p354 and E. coli_p350 (1:10; OD600 20).
Biotransformations were analysed by 1
H NMR relative to an internal standard of
trimethoxybenzene or by HPLC relative to an internal standard of caffeine.
All data are presented as mean values ± s.d. of three biological replicates. Credit: Nature
The most striking demonstration came when the researchers engineered E. coli to convert the Lossen product (PABA) into paracetamol via a two-enzyme pathway. By optimizing gene expression and fermentation conditions, they achieved high yields of paracetamol from both pure and PET-derived substrates (Figure 4). The process was robust, scalable, and did not require any fossil-derived chemicals.
Conclusion: Toward a New Era of Biocompatible Chemistry
This study marks a milestone in the merger of synthetic chemistry and biotechnology. By demonstrating a biocompatible Lossen rearrangement in living E. coli, the authors have opened new avenues for sustainable manufacturing, plastic upcycling, and metabolic engineering. The ability to convert plastic waste into essential medicines using engineered microbes is a compelling vision for the future of green chemistry and synthetic biology.
While further optimization and scale-up are needed, the approach is broadly applicable and could be extended to other reactions and products. The work stands as a testament to the power of interdisciplinary research and the promise of biocompatible chemistry in addressing global challenges.

A Biocompatible Lossen Rearrangement in Escherichia coli: Turning Plastic Waste into Medicine
A biocompatible Lossen rearrangement in Escherichia coli