A new study in Microsystems & Nanoengineering introduces a microneedle tip architecture aimed at a common failure in microinjection workflows: clogging during penetration and delivery.
The team led by Sarker and Sochol 3D prints monolithic, 650 micrometer tall hollow microneedles with two-photon direct laser writing (DLW). The design replaces the traditional tip orifice with multiple side ports, adds a sealed fine point, and integrates an internal microfilter. In live zebrafish embryo injections, the result is more consistent delivery and no complete blockages across extensive serial runs.
Microinjection supports applications from embryo manipulation to intraocular dosing. Conventional glass-pulled micropipettes are reliable and widely available, but their single axial opening aligns with the direction of travel.
Cellular or yolk material can lodge in that opening during puncture, reducing delivered volume, increasing variability, and sometimes stopping flow entirely. Side-port designs have been explored via cleanroom microfabrication, yet complexity and cost have limited adoption. High-resolution additive manufacturing changes the calculus by making intricate 3D tip features practical at scale.
Key Takeaways
- Architecture matters: a sealed fine point plus multiple side ports oriented perpendicular to insertion reduces penetration-induced clogging.
- Integrated microfilter prevents back-end debris from entering the thin tip channel, protecting side ports from occlusion.
- Two-photon direct laser writing enables 15 micrometer outer diameter, 10 micrometer inner diameter, high aspect ratio tips with submicron control.
- In vivo zebrafish embryo experiments show higher mean delivery and zero complete clogs for the side-port design across 1,000 injections per group.
- Compared to both glass and 3D-printed control tips with a single top opening, side-port tips delivered more consistently over 100 serial injections.
Overview
The study addresses the root cause of clogging in standard microinjection needles: alignment of the opening with the puncture direction. In the reported design, the tip apex is sealed, flow exits through twenty side ports distributed around the tip, each approximately 5 micrometers in diameter, positioned immediately below the apex.
This forces any biological material to travel orthogonally and occlude many discrete paths simultaneously to cause a full blockage, which is unlikely in practice. Upstream of the needle shaft, the authors pattern a curvilinear microfilter with approximately 3.25 by 3.25 micrometer pores to intercept aggregates that could otherwise block the narrow 10 micrometer inner channel.
Two-photon DLW makes it possible to realize this geometry. The process scans a tightly focused femtosecond infrared laser in a photosensitive resist, initiating localized two-photon polymerization with voxel dimensions around 0.6 micrometer laterally and 3.3 micrometers axially.
Using an ex situ workflow, the team prints the tip directly onto a fused silica capillary, achieving a fluid-tight, monolithic assembly in roughly ten minutes per side-port tip (about five minutes for a control tip lacking side ports). This approach bypasses the rectangular cross sections and limited aspect ratios common to traditional microfabrication, while eliminating manual tip opening steps that introduce variability.
To isolate the effect of tip geometry, the authors compare three needle types: conventional laboratory glass-pulled micropipettes, a 3D-printed control tip with a single 10 micrometer top opening, and the 3D-printed side-port tip.
Both 3D-printed tips share the same 15 micrometer outer and 10 micrometer inner diameters and include the microfilter. Dimensional measurements across multiple prints show markedly lower tip-to-tip variability for the 3D-printed needles than for hand-opened glass needles, suggesting future reductions in per-needle calibration time.
Methods
The authors print both tip designs in IP-L photoresist directly atop amber fused silica capillaries (inner diameter 75 micrometers; outer diameter 360 micrometers). The side-port tip includes twenty ~5 micrometer ports immediately below a sealed apex; both side-port and control tips incorporate a curvilinear microfilter at the base with ~3.25 by 3.25 micrometer pores.
In vitro, they assess tip-to-capillary seal integrity via flow rate versus input pressure and burst tests (monotonic and cyclic).
In vivo, they perform serial microinjections into manually dechorionated zebrafish embryos using fluorescein-labeled dextran, tracking delivery across 100 serial injections per needle (n = 1000 injections per group). They bracket each block of 20 embryo injections with pseudo-injections onto oil to quantify delivered droplet volumes and detect clogging.
Results
Printed tips meet geometric targets with tight distributions: ~15.3 ± 0.1 micrometer outer diameters and ~10.3 ± 0.1 micrometer inner diameters (n = 10 each), substantially more repeatable than hand-opened glass tips. Micrographs show clean attachment and well-formed side ports; sectioned tips confirm microfilter pore size smaller than side ports, reducing downstream blockage risk.
In vitro, flow rate scales linearly with pressure and no burst failures occur under tested conditions for either control or side-port assemblies. In vivo, side-port tips deliver higher mean fluorescence than both glass and 3D-printed control tips, most pronounced in the first 20 injections: 6.45 ± 1.63 (side-port) vs. 4.52 ± 1.58 (glass, p < 0.0001) and 5.73 ± 1.38 (3D control, p < 0.001). Across later blocks, side-port tips maintain an advantage and avoid the steep declines seen in the other groups.
Pseudo-injection assessments corroborate the anti-clogging effect. Glass tips show substantial declines in normalized delivered volume and frequent complete occlusions with an overall complete clogging rate of 44.0 ± 26.3%. 3D-printed control tips improve on this with 26.0 ± 23.2% complete clogs. In contrast, side-port tips exhibit no complete clogging events across all assessments.
Why It Matters
Clogging-induced failures degrade microinjection throughput and reproducibility, and in some settings, compromise outcomes that depend on reliable doses. For workflows with limited biological material, long serial runs, or critical payloads, a blocked needle is costly. By demonstrating a practical, manufacturable architecture that mitigates clogging without increasing needle diameter, this work broadens the design space for microinjection tools and shows how fine-scale additive manufacturing translates to better bench performance. The approach could standardize dosing, reduce downtime, and lower training burden.
Beyond zebrafish, microinjection is foundational to embryo manipulation, gene editing, and intra-organ dosing. Zebrafish offer a well-characterized benchmark for staging and survival (Kimmel et al., 1995). Hollow microneedles are also used for transdermal delivery and biosampling, where side-opened geometries have shown promise (Cárcamo Martínez et al., 2021). The reported printing strategy should generalize to other targets and payloads where anti-clogging behavior is valuable.
What the Figures Show
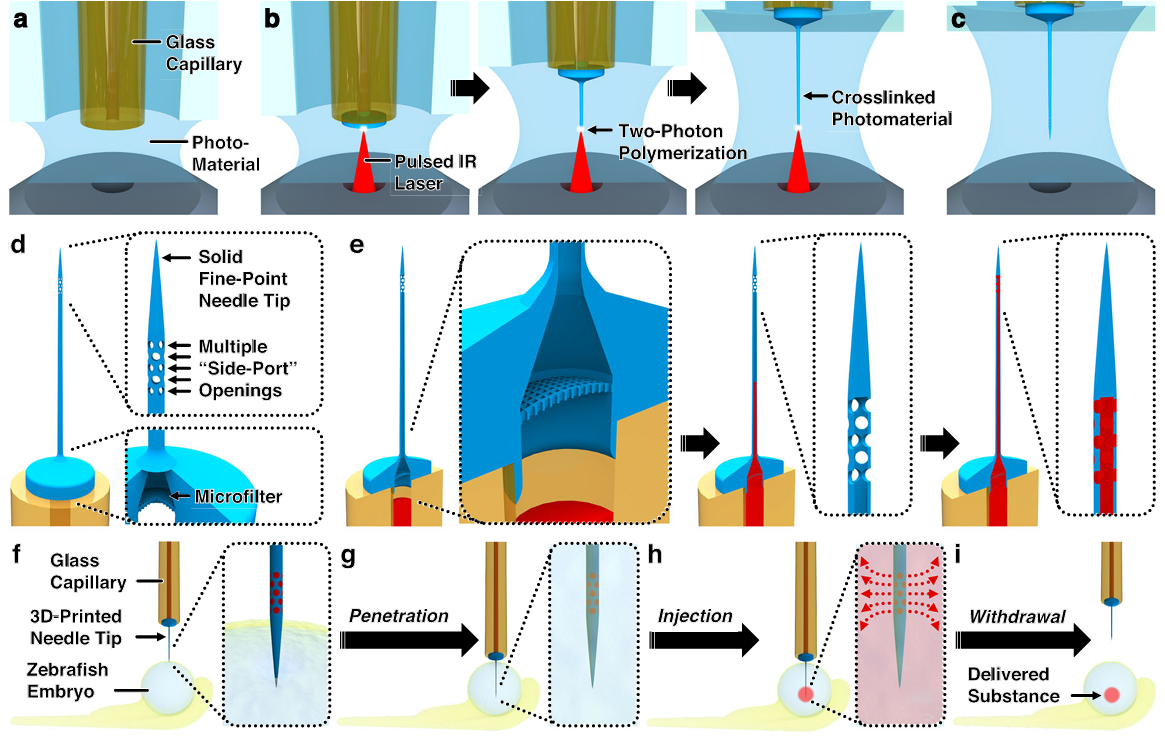
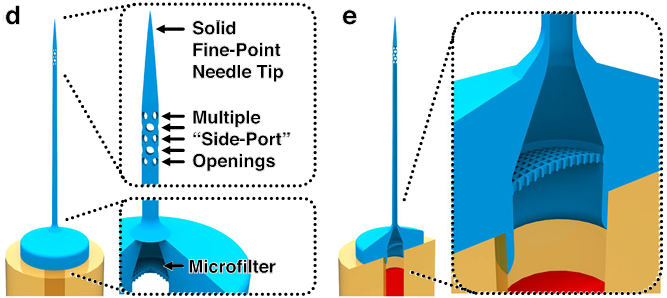
Fig. 1 Conceptual overview of using “ex situ Direct Laser Writing (esDLW)” to additively manufacture hollow microneedles with 3D architectures for embryo microinjections. a–c The esDLW strategy for printing the monolithic 3D microneedle tip. a A glass capillary—e.g., an amber-fused silica tube—loaded into a DLW 3D printer with one end enveloped in photosensitive material. b A tightly focused femtosecond infrared (IR) laser is scanned point by point, layer by layer within the photosensitive material to initiate two-photon polymerization (2PP) in designated locations directly atop the capillary. c The completed needle tip fluidically sealed to the capillary. d Key DLW-enabled 3D architectural needle tip features. e The microfluidic loading and delivery path in which a fluid/suspension passes: (left) from the capillary into the printed needle tip through the internal microfilter, (middle) through the thin main channel, and then (right) out of the tip via multiple “side-port” openings (i.e., perpendicular to the main channel). f–i Key steps for zebrafish embryo microinjections. f The solid, fine-point tip puncturing the surface of the embryo. g The microneedle penetrating into the embryo. h Microinjection-based delivery of foreign substances into the embryo via the microneedle tip’s multiple side-port openings. i Withdrawal of the microneedle from the embryo, leaving behind the delivered substance.
Figure 1 provides a conceptual overview. The ex situ DLW sequence immobilizes a fused silica capillary in photoresist, then writes the tip point-by-point, layer-by-layer. The printed structure includes three hallmark features: a sealed fine point, an annulus of side ports immediately below the apex, and a base microfilter adjacent to the capillary interface. The flow path forces suspensions to traverse the filter, then the narrow tip channel, and only then exit through multiple ports. During penetration, material at the target surface cannot enter a sealed apex, eliminating common lodgment failures seen with single opening tips.
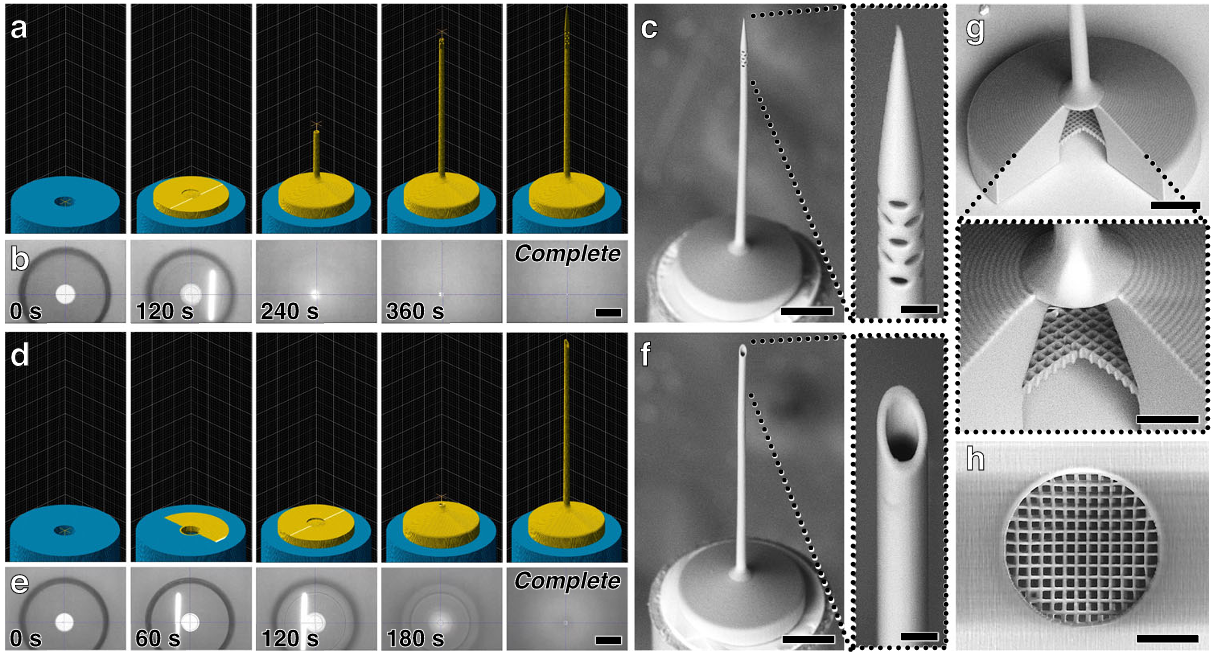
Fig. 2 Fabrication results for esDLW-based 3D printing of microinjection needles directly atop fused silica glass capillaries. a–c 3D needle tip designs. d–f Control tip designs. a, d Computer-aided manufacturing (CAM) simulations, and b, e corresponding micrographs captured during the esDLW printing process. Scale bars =100 µm (See also Supplementary Movie S1; Supplementary Movie S2). c, f Scanning electron microscope (SEM) imagesofrepresentativeprintingresults.Scalebars= 100 µm (Expanded views =10 µm). g,h SEM micrographs of printed integrated microporous f ilters. g Sectioned view. Scale bar =100 µm (Expanded view =25µm). h Bottom view. Scale bar=25µm
Figure 2 documents fabrication and geometry. The authors report ~15.3 ± 0.1 micrometer outer diameters and ~10.3 ± 0.1 micrometer inner diameters across ten 3D-printed tips, with similar means for the control tips, and significantly tighter distributions than conventional glass tips. Low-vacuum SEM and optical micrographs show clean attachment to the capillary and well-formed side ports at approximately 5 micrometers. Sectioned tips reveal microfilter pores around 3.25 by 3.25 micrometers, smaller than the side ports, which reduces the likelihood of downstream blockage.
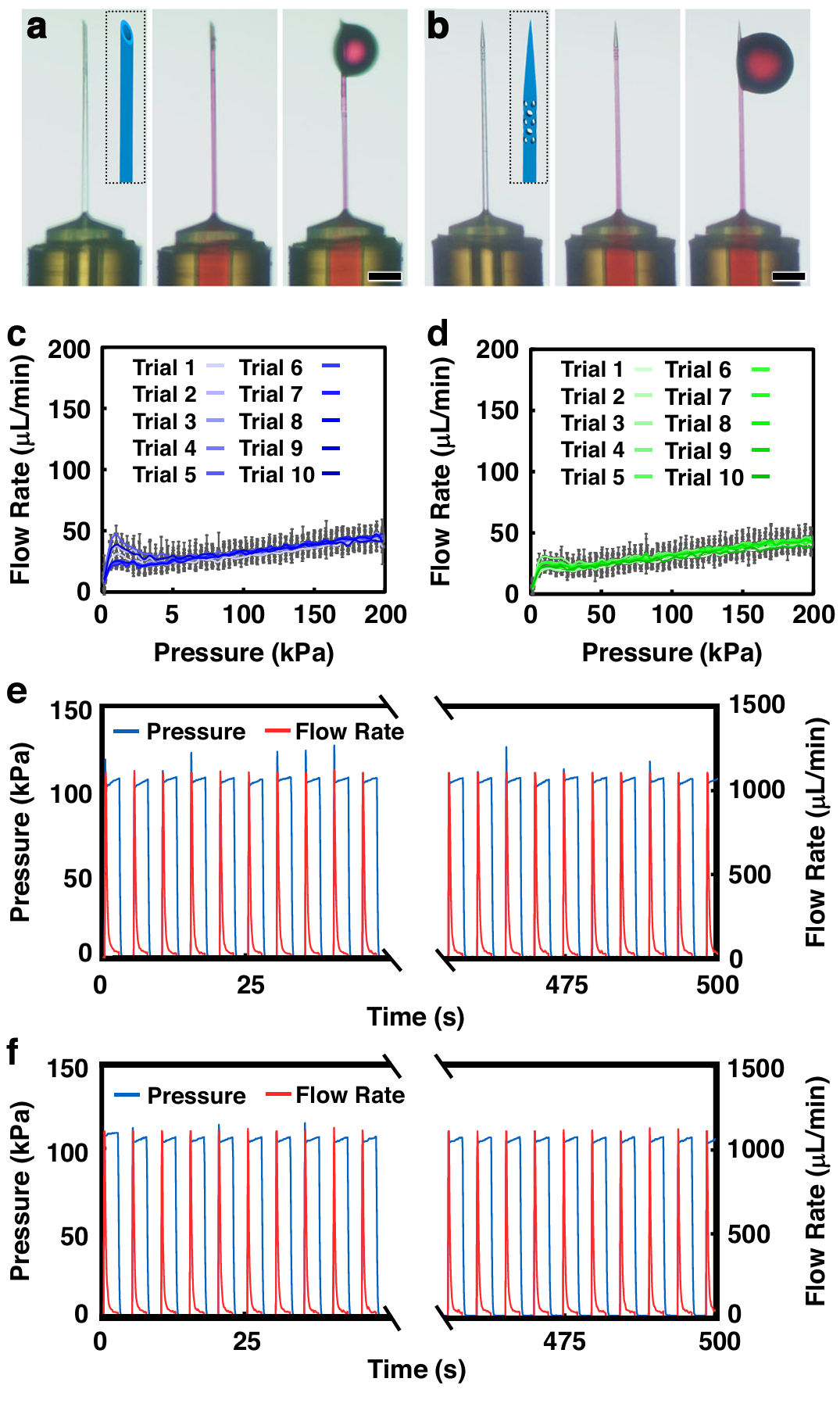
Fig. 3 Experimental results for microfluidic investigations of print-capillary interface integrity. a,b Sequential images of fluidic infusion of rhodamine-dyed DI water into esDLW-printed microinjection needles with a control, and b 3D needle tip designs. Scale bars =100µm. c,d Linear microfluidic burst-pressure results for esDLW-printed microinjection needles with c control, and d 3D needle tip designs (n =10 distinct needles per design). Error bars = S.D. e,f Representative cyclic microfluidic burst-pressure results for n = 100 ramping cycles for esDLW-printed microinjection needles with e control, and f 3D needle tip designs
Figure 3 assesses the integrity of the printed tip-to-capillary seal under pressure. Flow rate versus input pressure traces are linear and consistent, and the assemblies withstand both monotonic and cyclic burst tests at pressures exceeding those used in zebrafish injections. No burst failures are observed for either control or side-port tips under the tested conditions. These in vitro data establish that the monolithic seal is robust; delivery differences in vivo are not explained by leakage or interface failure.
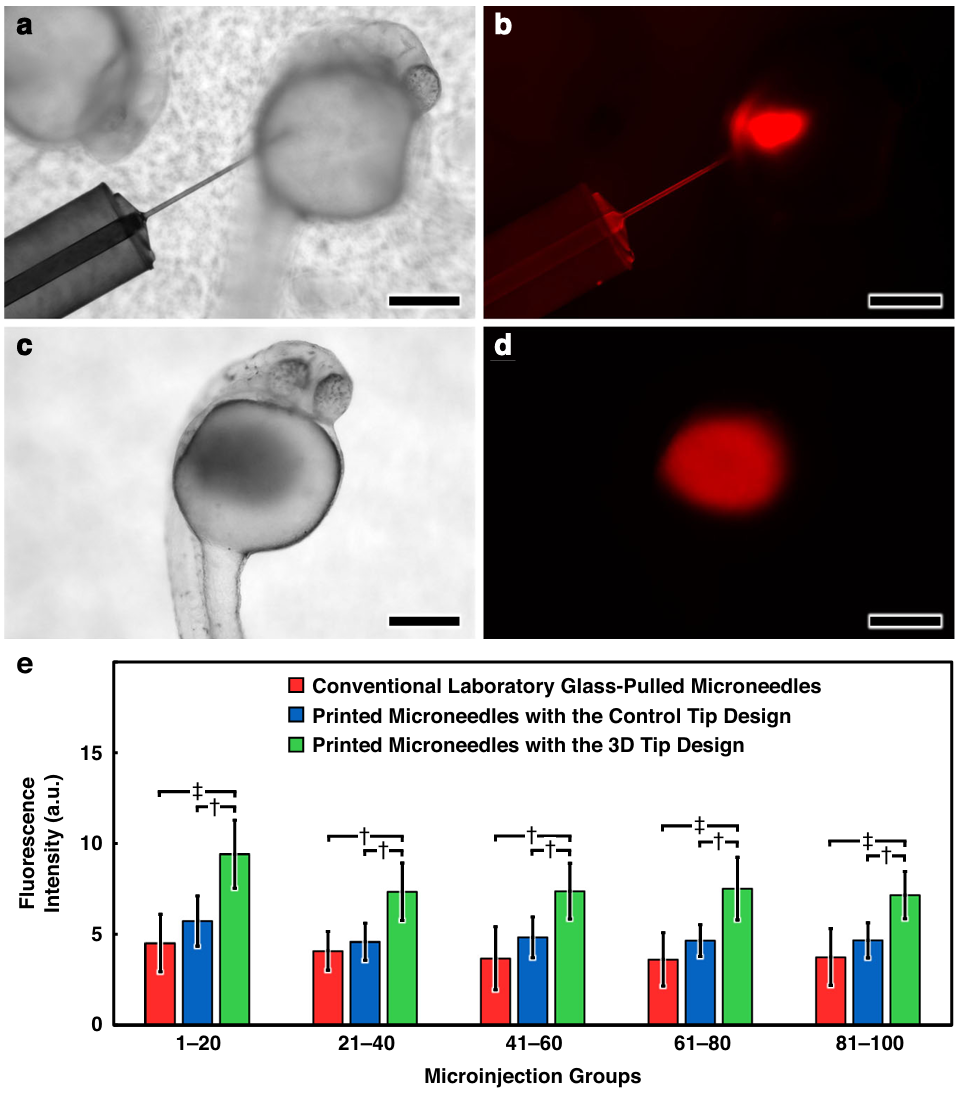
Fig. 4 Experimental results for microinjections of fluorescently labeled aqueous fluid into live dechorionated zebrafish embryos in vivo. a–d Sequential micrographs a, b during, and c, d after delivery of rhodamine B-dyed DI water into an embryo using an esDLW-printed microneedle with a 3D tip design imaged under a, c brightfield, and b, d fluorescence microscopy. Scale bars= 250µm. e Quantified fluorescence intensity of groups of lysed zebrafish embryos following microinjection with various needle types (n = 10 distinct microneedles per needle type with 100 microinjections per needle; N=3000 microinjections in total). Error bars = S.D.; † and ‡ denote p <0.001 and p<0.0001 statistical significance, respectively
Figure 4 covers in vivo performance. Using fluorescein-labeled dextran injections into manually dechorionated embryos, the authors quantify delivered signal after each block of twenty injections over one hundred serial injections per needle type (n = 1000 per group). While the glass and 3D control tips are not statistically distinguishable, the side-port tips deliver higher mean fluorescence, most notably in the first twenty injections: 6.45 ± 1.63 vs. 4.52 ± 1.58 (glass, p < 0.0001) and 5.73 ± 1.38 (3D control, p < 0.001). Across later blocks, the side-port tips maintain an advantage and avoid steep declines seen in the other groups.
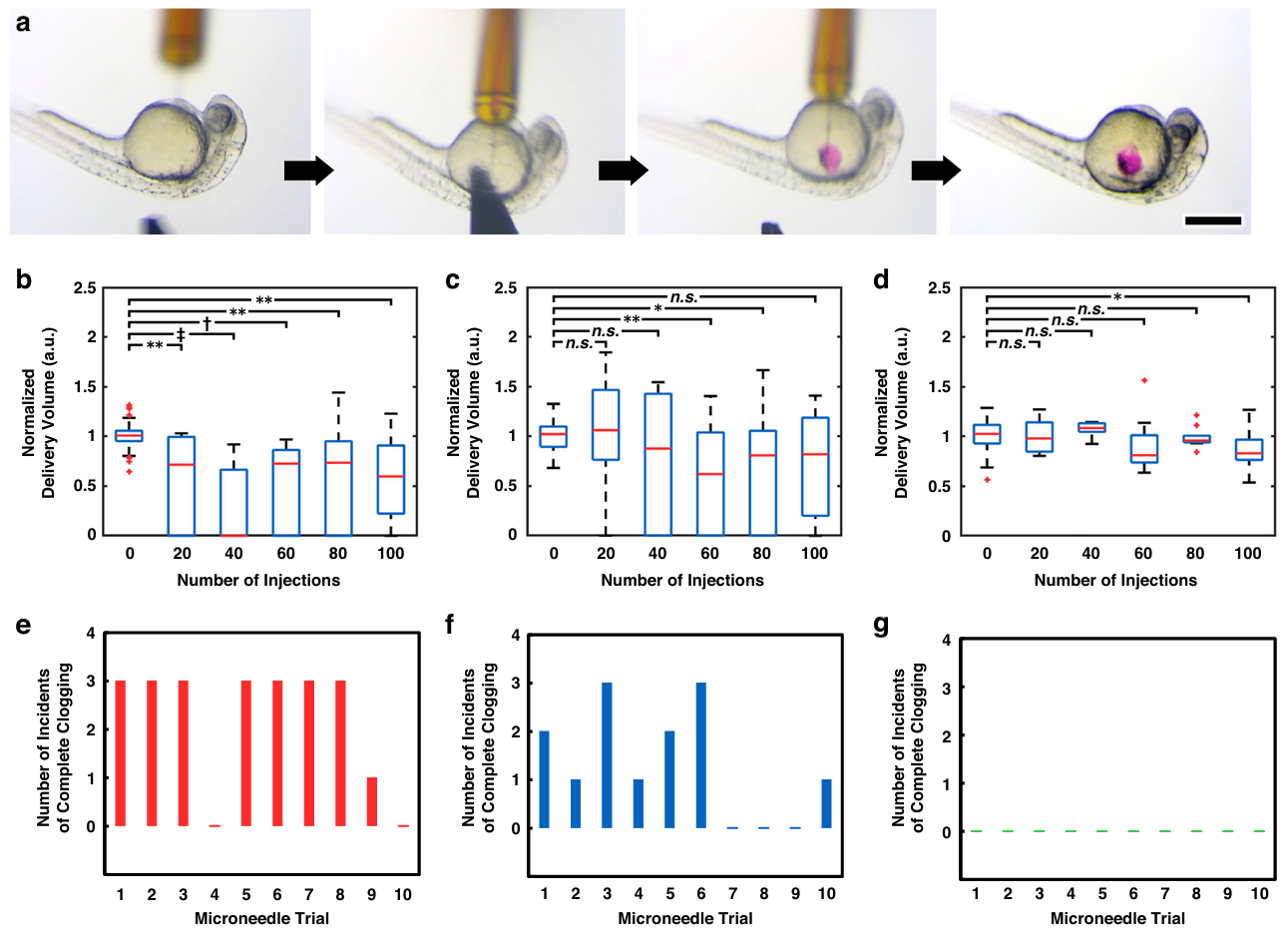
Fig. 5 Experimental results for clogging phenomena associated with microinjections into live dechorionated zebrafish embryos for various microneedle types. a Sequential micrographs of the embryo microinjection process using an esDLW-printed microneedle with a 3D tip design. Scale bar =500 µm; See also Supplementary Movie S3. b–d Normalized delivery volumes measured at 20-injection increments over the course of serial injection trials comprising 100 zebrafish embryo microinjections for: b conventional laboratory glass-pulled microneedles, c esDLW printed microneedles with the control tip design, and d esDLW-printed microneedles with the 3D tip design (n=10 distinct microneedles per needle type). *, **, †, and ‡ denote p < 0.05, 0.01, 0.001, and 0.0001 statistical significance, respectively; n.s. = not significant. e–g Number of incidents of complete microneedle clogging (i.e., cases in which no fluid was delivered during a pseudo-injection attempt) per trial examined at 20-injection increments (n =5 delivery volume assessments per trial) over the course of serial injection trials comprising 100 microinjections for: e conventional laboratory glass-pulled microneedles, f esDLW-printed microneedles with the control tip design, and g esDLW-printed microneedles with the 3D tip design (n =1 distinct microneedle per trial; 10 distinct microneedles per needle type)
Figure 5 probes clogging directly via pseudo-injections. Glass tips exhibit substantial declines in normalized volume after embryo injections and high rates of complete occlusion events, with 44.0 ± 26.3% complete clogging. 3D-printed control tips improve upon this at 26.0 ± 23.2% but still show frequent clogs. In contrast, the side-port tips show minimal decline in pseudo-injection volume and no complete clogs across all assessments, indicating that the architecture sustains delivery over long serial runs.
Two caveats are notable.
First, improvement likely stems from both the side-port orientation and manufacturing precision. Even the 3D-printed control tips, which retain a single top opening, show tighter initial dose distributions than hand-opened glass tips.
Second, side ports may alter local flow fields and tissue interactions; while embryo survival and qualitative post-injection images are reported, systematic assessment of off-target effects and tissue damage in other models would strengthen generalization.
Practical Guidance
When adopting side-port microneedles, match port size and count to payload size distribution and viscosity. The integrated microfilter should be selected to block aggregates larger than downstream features.
For highly particulate or viscous formulations, consider pre-filtration and short pulses to reduce transient occlusion. Validate dose uniformity with pseudo-injections before long serial runs. Sterilization should preserve tip geometry; avoid harsh polishing or solvent swelling that could alter port dimensions.
Conclusion
Sarker and colleagues show that tip architecture, not just diameter, governs real-world microinjection reliability. Combining a sealed apex, multiple side ports, and an integrated microfilter in a monolithic 3D-printed tip reduces variability and eliminates complete clogs across extensive zebrafish embryo injection series.
Two-photon DLW provides the resolution to place features where they matter and the repeatability to reduce calibration overhead. Promising extensions include adapting port patterns for different targets, tailoring filters to payloads, and assembling multi-needle arrays for high throughput. For labs that rely on microinjection, this work offers an actionable, manufacturable template that performs measurably better in use (Sarker et al., 2025).
Definitions
Two-Photon Direct Laser Writing: An additive manufacturing process that uses a focused femtosecond infrared laser to initiate simultaneous absorption of two photons in a photoresist, polymerizing only at the focal voxel for submicron 3D feature control (Nanoscribe, 2025).
Side Port Microneedle: A hollow needle whose outlet openings are oriented orthogonally to the insertion direction, here arranged as multiple circular ports immediately below a sealed apex.
Microfilter: A porous structure integrated at the needle base that screens out particulates larger than its pore size to prevent downstream occlusion. In this work, pores are approximately 3.25 by 3.25 micrometers.
Zebrafish Embryo Microinjection: Delivery of reagents into zebrafish embryos at defined developmental stages used for gene function studies, lineage tracing, and disease modeling (Kimmel et al., 1995).
Hollow Microneedles: Needles with internal channels for fluid flow used in transdermal delivery and biosampling; side-opened geometries can reduce clogging in tissue (Cárcamo Martínez et al., 2021).
References
Primary study: (Sarker et al., 2025). Background and definitions: (Nanoscribe, 2025); (Cárcamo Martínez et al., 2021); (Kimmel et al., 1995).

3D Nanoprinted Microneedles That Resist Clogging In Embryo Microinjection
3D Nanoprinted Microneedles That Resist Clogging In Embryo Microinjection