What if plastic waste didn't end up in landfills or oceans but instead becomes the starting point for life-saving medicines. Researchers researchers are working towards this reality by integrating the classic Lossen rearrangement, a reaction previously restricted to the chemistry lab, inside living Escherichia coli cells. This research expands what microbes can accomplish and offers hope for more sustainable chemical manufacturing.
Advancing the Field of Biocompatible Chemistry
Traditional chemical synthesis and biological processes have long operated in separate spheres. Synthetic chemists can perform reactions beyond the reach of nature, while living organisms are bound by evolutionary limits.
Biocompatible chemistry bridges this divide, introducing non-natural reactions into living cells and unlocking new possibilities for biomanufacturing.
- The Lossen rearrangement, which converts carboxylic acids to primary amines, was achieved inside E. coli for the first time.
- Phosphate ions found naturally in cells catalyzed the reaction under mild, cell-friendly conditions.
- This is the first integration of the Lossen rearrangement with microbial metabolism transforming a purely chemical reaction into a biological process.
Upcycling Plastics into Valuable Chemicals
One major application of this innovation is plastic upcycling. The researchers created the Lossen rearrangement starting material from polyethylene terephthalate (PET), the plastic in common beverage bottles.
Engineered E. coli then converted this PET-derived molecule into para-aminobenzoic acid (PABA), an essential compound for life and a key precursor to pharmaceuticals.
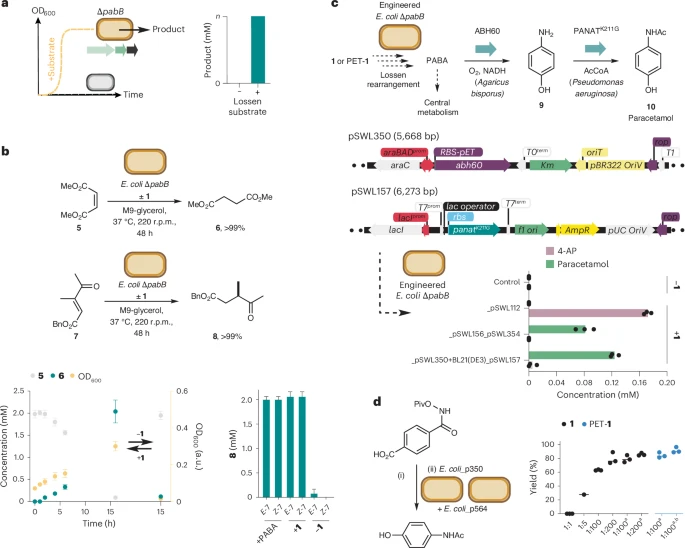
- Through an auxotroph rescue experiment, E. coli strains unable to produce PABA were supplied with the PET-derived substrate and successfully grew, proving the reaction’s functionality inside living cells.
- This strategy demonstrates the potential to use plastic waste as a feedstock for both microbial growth and the production of valuable chemicals.
a, Interfacing the Lossen rearrangement with native and engineered biosynthetic pathways in E. coli. b, Biotransformation of DMM (5) to dimethylsuccinate (6) and β-ketoacrylate 7 to γ-ketoester 8 using E. coli BW25113∆pabB and 1 or PET-1. c, A de novo biosynthetic pathway to 4-AP (9) and paracetamol 10 incorporating a non-enzymatic Lossen rearrangement, plasmid designs and whole-cell production experiments. d, Paracetamol synthesis by one-pot Lossen rearrangement and bacterial whole-cell synthesis. Strain E. coli_p350 expresses ABH60 and strain E. coli_p354 expresses PANATK211G. Ratios refer to E. coli_p354 and E. coli_p350 in biotransformations (1:1, OD600 26; 1:5, OD600 16; 1:100 and 1:200, OD600 12.5). aFinal cell density OD600 25. OD refers to the final optical density at 600 nm. bCells induced with 0.5% l-arabinose. Reaction conditions: (i) substrate (0.5 mM), aqueous potassium phosphate (200 mM, pH 8.0), 50 °C, 48 h; (ii) E. coli_p354 and E. coli_p350 (1:10; OD600 20). Biotransformations were analysed by 1H NMR relative to an internal standard of trimethoxybenzene or by HPLC relative to an internal standard of caffeine. All data are presented as mean values ± s.d. of three biological replicates.
Engineering Microbes for Medicine Production
The research went a step further by engineering E. coli to transform PABA into paracetamol (acetaminophen), a widely used pain reliever.
By introducing two key enzymes, scientists established a de novo pathway, one not found in nature, that converts the intermediate into the final pharmaceutical product.
- Enzymes aminobenzoate hydroxylase and arylamine N-acyltransferase were added to enable this transformation.
- This two-step process, combining chemical and enzymatic methods, achieved high yields of paracetamol from PET-derived starting materials.
- The approach exemplifies the power of merging synthetic biology with non-natural chemistry to manufacture medicines from waste streams.
The Future of Sustainable Chemical Manufacturing
This pioneering study suggests several exciting implications for chemistry and biotechnology:
- Broader metabolic capabilities: Biocompatible reactions give engineered microbes access to new chemical transformations outside the reach of natural metabolism.
- Plastic remediation and circular economies: Turning waste PET into high-value products offers a dual benefit for the environment and industry.
- Integration with metabolic engineering: Linking non-natural reactions to cellular growth and product formation opens up new avenues for biocatalysis.
- Accelerated innovation: Combining chemical and biological tools speeds up the development of sustainable production systems without relying solely on enzyme engineering.
Takeaway
This research marks a leap forward in chemical biotechnology. By successfully integrating a classic chemical reaction into living cells, scientists have paved the way for converting plastic waste into essential medicines through engineered microbes.
As the boundaries between chemistry and biology continue to blur, these hybrid strategies promise a more sustainable, circular future for manufacturing and environmental stewardship.
Be sure to checkout the original publication for all the details on this exciting development!
Source: Nature Chemistry (2025), "A biocompatible Lossen rearrangement in Escherichia coli" by Nick W. Johnson et al. Read the original article.

Turning Plastic Waste into Medicine: Biocompatible Chemistry Breakthrough in E. coli
A biocompatible Lossen rearrangement in Escherichia coli