Artificial intelligence is reshaping drug discovery, moving well beyond predicting static protein structures to modeling the intricate dance of biomolecular interactions. This leap is enabling scientists to target disease pathways once deemed untreatable and unlock new classes of therapeutics.
The Power of Protein Interactions
Biology’s real complexity lies in the interactome, the vast network of protein-protein interactions (PPIs) that regulate cellular life. When these networks malfunction, diseases such as cancer or neurodegeneration can result.
Historically, PPIs challenged drug developers because their large, featureless surfaces resisted conventional small-molecule drugs. Thanks to AI, researchers are now able to pinpoint and design molecules to modulate these critical protein interfaces, reviving tumor suppressor pathways or restoring cell death mechanisms in cancer therapy.
AlphaFold’s Evolution: Modeling the Unseen
AlphaFold2 revolutionized science by predicting millions of protein structures, but it couldn’t account for dynamic behaviors or complex molecular assemblies. AlphaFold-Multimer improved on this by handling protein complexes, including disordered segments, yet still fell short with non-protein partners.
The arrival of AlphaFold 3 changed the game. Using a diffusion-based generative model, it predicts structures of assemblies involving proteins, DNA, RNA, and small molecules with unprecedented accuracy, outpacing traditional methods. While the most powerful version remains proprietary, its public release signals a major advance in rational drug design.
A Thriving AI Tool Ecosystem
AlphaFold may have set the standard, but a growing ecosystem of AI tools is tackling drug discovery’s many challenges:
- PIONEER forecasts how pathogenic mutations disrupt protein networks, aiding the hunt for new drug targets.
- FragFold engineers small protein fragments from native sequences, creating genetically encodable therapies.
- PINNACLE contextualizes PPIs within specific tissues or cell types for greater biological relevance.
- Graph Neural Networks, Large Language Models, and Multimodal AI synthesize data to predict molecular interactions, binding affinities, and even systemic effects, often with user-friendly language interfaces.
Real-World Impact: From Bench to Bedside
AI-driven pipelines are rapidly translating computational predictions into real-world therapies. In oncology, companies like Iambic Therapeutics and Relay Therapeutics design highly selective drugs that minimize side effects by targeting disease-specific proteins.
In infectious disease, AI has already enabled the discovery of novel antivirals and accelerated antibody development. Major partnerships, such as between Isomorphic Labs and Eli Lilly, highlight the commercial and therapeutic value of these advances.
The companies developing these therapies represent a new breed of biotech, where computational power is as central as the wet lab.
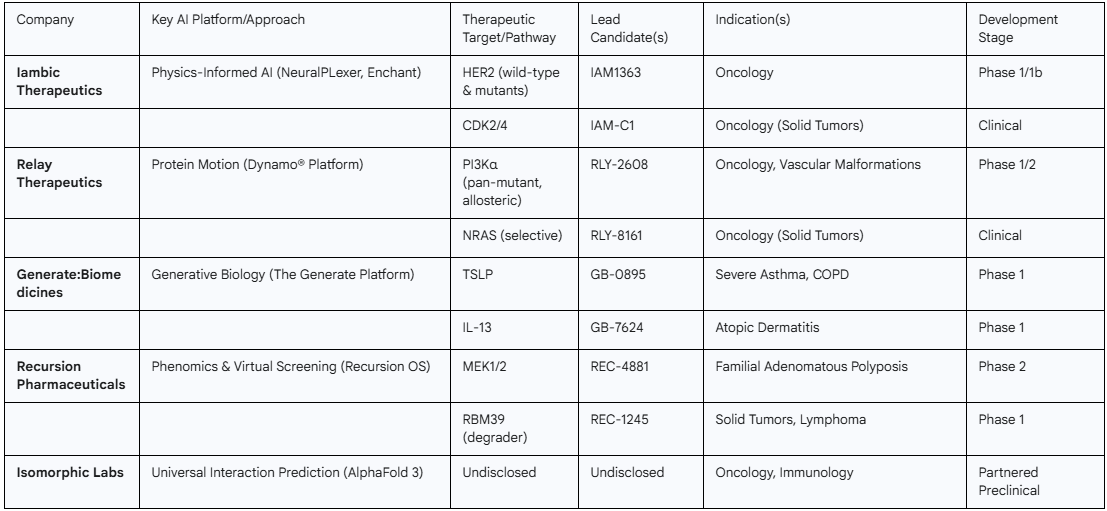
- Isomorphic Labs: As the commercial arm of DeepMind, Isomorphic Labs wields the full, unrestricted power of AlphaFold 3. While their internal pipeline remains largely under wraps, they have publicly stated a focus on oncology and immunology and have forged massive, multi-billion dollar collaborations with Eli Lilly and Novartis to discover novel small molecules against undisclosed targets.
- Generate:Biomedicines: Using its proprietary "Generate Platform," this company is a leader in generative biology for creating novel protein therapeutics. Their public pipeline is heavily weighted towards Immunology & Inflammation, with programs targeting key cytokines like TSLP (for asthma), IL-13 (for atopic dermatitis), and TL1A (for ulcerative colitis).
- Iambic Therapeutics: Iambic's platform combines physics-informed AI models for structure prediction (NeuralPLexer) and property prediction (Enchant). Their strategy is clearly focused on delivering best-in-class small molecules in oncology, with their HER2 and CDK2/4 inhibitors already in the clinic.
- Relay Therapeutics: Differentiating itself with a focus on protein motion, Relay's Dynamo® platform is designed to drug targets that are intractable with static structural methods. Their pipeline reflects this, with candidates against challenging targets like PI3Kα and NRAS in oncology, as well as a program for the genetic disorder Fabry disease.
- Recursion Pharmaceuticals: Recursion's strength lies in its vast, proprietary biological and chemical dataset, which it uses to build "Maps of Biology." Its AI models, like MatchMaker, perform massive virtual screens (e.g., predicting interactions for 36 billion compounds) to identify novel drug-target relationships. Their pipeline includes inhibitors for
MEK1/2 and CDK7, and a degrader for RBM39. - Elix, Inc. & PRISM BioLab: This partnership exemplifies a highly specialized strategy. It combines PRISM's unique PepMetics® chemistry platform, which creates small molecules that mimic peptide secondary structures like alpha-helices, with Elix's AI discovery engine. Their explicit goal is to tackle challenging PPI targets in cancer, fibrosis, and autoimmune disease that have resisted other approaches.
Current Challenges and Ethical Hurdles
- Theory vs. Practice: AI outputs are hypotheses needing experimental validation in the lab.
- Data Quality and Transparency: Biased training data and opaque deep learning models require careful oversight and regulatory adaptation.
- Access and Equity: Proprietary models and high computational costs risk deepening divides between industry and academia.
- Ethical and Regulatory Frameworks: As AI-designed drugs enter clinics, robust guidelines for integrity, explainability, and fair access become essential.
The Future: Dynamic, Human-Centered Discovery
Looking ahead, the next generation of AI tools will be even more dynamic, context-aware, and multimodal, integrating protein movement, cellular context, and diverse biological data.
Success may very will depend on "human-in-the-loop" strategies, where AI tackles scale and complexity, and scientists guide and validate results. For R&D leaders, agile cycles of prediction and lab testing will be key. Investors should focus on platforms with validated predictions, strong data assets, and interdisciplinary teams.
AI’s journey from protein folding to modeling molecular interactions is revolutionizing drug discovery, paving the way for precision therapeutics and a new era of biopharmaceutical innovation. Ensuring these breakthroughs benefit global health will require bridging computational insight with experimental rigor, transparency, and equitable access.

AI In Drug Discovery: From Protein Structures to Molecular Interactions