The increasing accumulation of plastic waste, particularly black polystyrene, has posed significant environmental challenges due to its poor recyclability. A recent NIH funded study by Oh et al. (2025) presents a groundbreaking method using photothermal conversion to efficiently depolymerize polystyrene into monomers, offering a viable pathway toward a circular plastic economy.
Key Takeaways
- The study demonstrates the use of photothermal conversion to depolymerize polystyrene plastics into styrene monomers using carbon black as a photothermal agent.
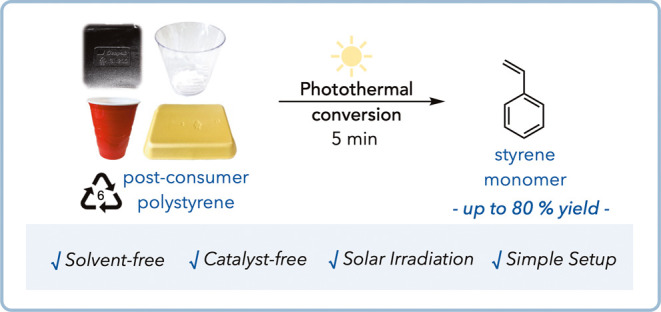
- The method achieves up to 60% yield of styrene monomer under white LED light irradiation and up to 80% yield using focused solar irradiation.
- The method works without the need for external catalysts or solvents, reducing processing complexity.
- This technique can be applied to unmodified post-consumer black polystyrene samples, making it a practical solution for recycling black plastics.
- The process is scalable and can be integrated into existing recycling infrastructure, offering a sustainable solution for plastic waste management and promoting a circular economy.
Overview
Photothermal conversion involves using nanomaterials or chromophores to convert light into thermal energy, which can then be used to drive chemical reactions. This process allows for localized heating, providing spatial and temporal control over reactions.
Traditional pyrolysis methods for chemical recycling of plastics require high temperatures, often leading to undesirable byproduct formation. Photothermal conversion, however, can achieve similar results at lower bulk temperatures, making it a more efficient and selective method for depolymerization.
Carbon black, a common additive in black plastics, acts as an effective photothermal agent. It enhances the physical properties of commercial plastics and has been shown to promote photothermal conversion for various organic reactions.
Despite its advantages, black plastics present unique recycling challenges due to their inertness to various stimuli and difficulties in sorting. This study addresses these challenges by leveraging the photothermal properties of carbon black to facilitate the depolymerization of black polystyrene plastics.
When irradiated, photothermal conversion agents are excited and undergo nonradiative decay, releasing heat localized to the nanoparticle surface while maintaining relatively low bulk temperatures.
The research begins by synthesizing polystyrene-carbon black (PS-CB) composites and studying their depolymerization under white LED light irradiation. The results show that increasing carbon black content leads to higher styrene monomer yields, with a maximum of 57% achieved at 5 wt% carbon black loading.
Further experiments demonstrate the depolymerization of post-consumer black polystyrene samples without additional catalysts or solvents, using natural sunlight can achieve monomer yields of up to 80%. The researchers show that this can be extended to non PS-CB plastics by simply combining black plastics with pure polystyrene.
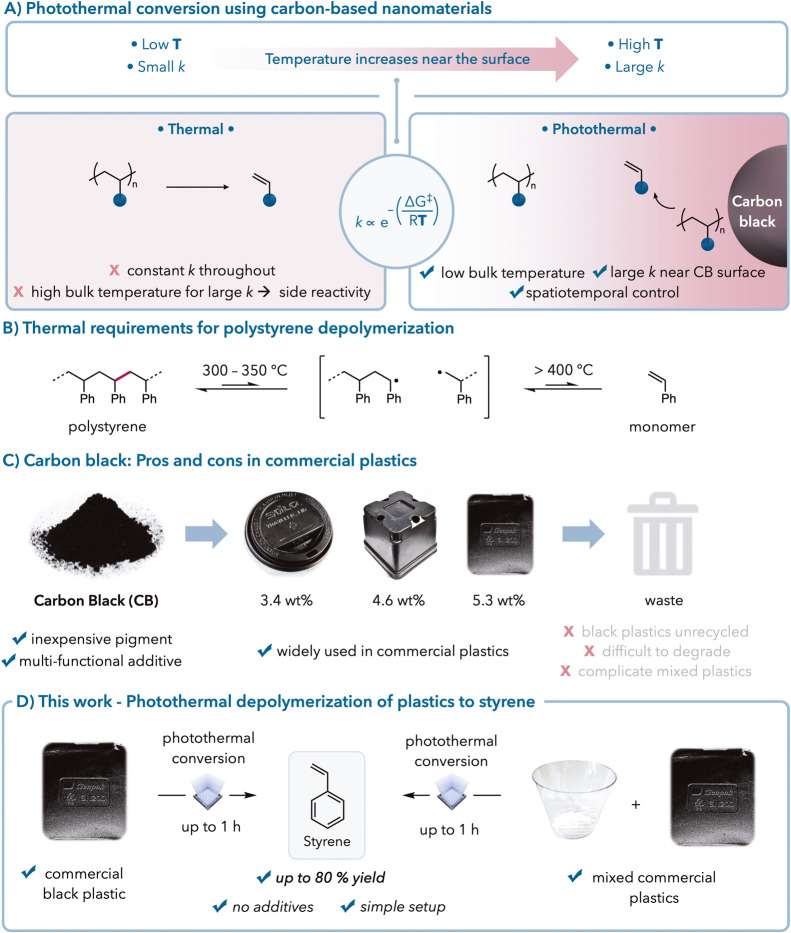
Using photothermal conversion to overcome existing recycling limitations. (A) Bulk heating versus photothermal conversion with localized heating. (B) Minimum polystyrene pyrolysis requirements. (C) Use of carbon black in existing plastics. (D) Photothermal conversion for chemical recycling to monomers of black and mixed commercial polystyrene plastics.
Why It's Important
This research has the potential to revolutionize plastic waste management. By utilizing photothermal conversion, the study offers a sustainable and efficient method for recycling black plastics, which are notoriously difficult to recycle using traditional methods. This technique can be integrated into existing recycling infrastructure, reducing the environmental impact of plastic waste and promoting a circular economy.
The use of carbon black as a photothermal agent is a cost-effective solution, as it is an inexpensive and commonly used additive in black plastics. The method's ability to depolymerize unmodified post-consumer non-black polystyrene samples in a mixture further emphasizes its practicality and potential for large-scale application.
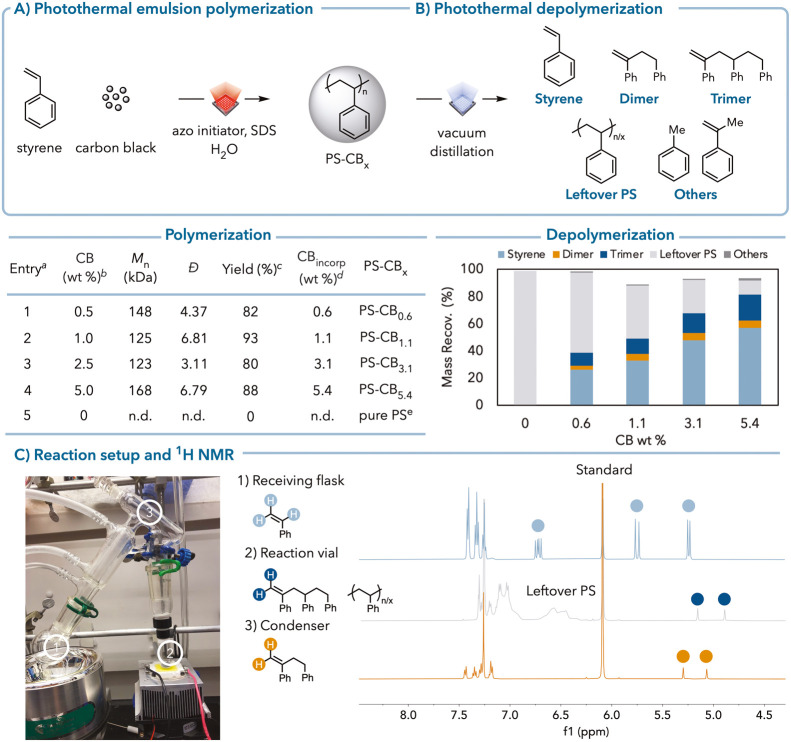
Photothermal polymerization and depolymerization. (A) Black polystyrene is synthesized via photothermal emulsion polymerization. (a) Detailed reaction conditions are provided in the Supporting Information. (b) CB wt % used in polymerization is calculated using the mass of CB/the mass of styrene. (c) Isolated yield of PS-CBx. (d) The calculation method for CB incorporated in PS is given in the Supporting Information. (e) Pure PS was synthesized via thermal emulsion polymerization at 80 °C. (B) PS-CBx (50 mg) is photothermally depolymerized to small molecules. The calculation method for mass recovery is in the Supporting Information. The values are based on a styrene repeat unit basis. (C) (Left) Picture of photothermal depolymerization setup. (Right) 1H NMR spectrum of the receiving flask, reaction vial, and condenser after photothermal depolymerization of PS-CB1.1.
Summary of Results
The study conducted several experiments to evaluate the efficiency and practicality of photothermal depolymerization of polystyrene using carbon black.
To achieve efficient depolymerization, the PS-CB samples were irradiated with high-intensity white LED light. This setup ensured that the local temperatures of carbon black exceeded the ceiling temperature (Tc) for styrene, which is approximately 395°C.
The depolymerization process yielded styrene monomer as the primary product, along with smaller amounts of dimer, trimer, toluene, and α-methylstyrene (AMS). The measured bulk temperatures during the process did not exceed 150°C, which is significantly lower than the degradation temperature (Td) for polystyrene.
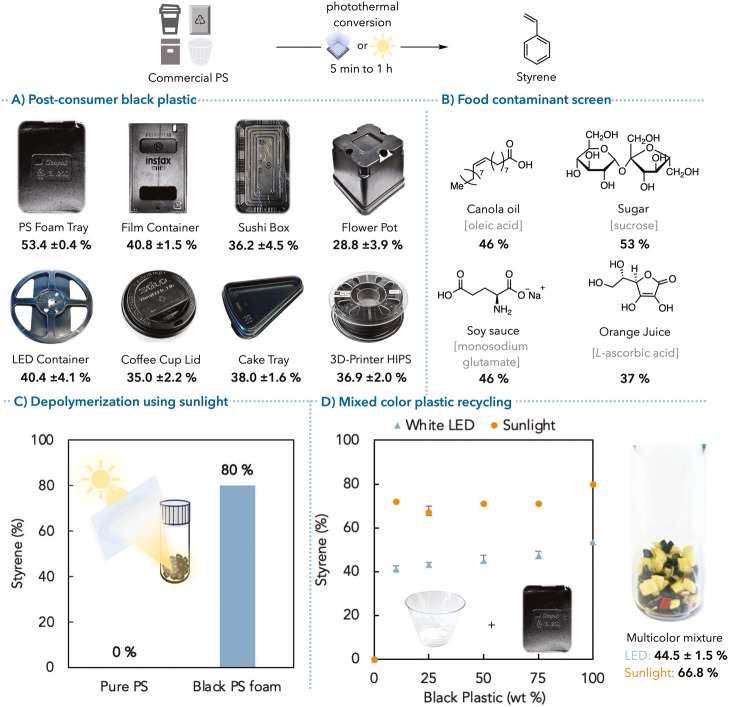
The study also explored the depolymerization of post-consumer black polystyrene samples, including foamed polystyrene (PS foam), food containers, and coffee cup lids. These samples were subjected to the same depolymerization conditions without the need for additional carbon black or other catalysts. The results demonstrated successful depolymerization with styrene yields of up to 53%.
They also investigated the use of focused solar irradiation as an energy source for photothermal depolymerization. Using a Fresnel lens, commercial PS samples were irradiated under static vacuum, achieving up to 80% styrene yield in just 5 minutes. This method was also applied to mixed-color post-consumer waste PS samples, yielding up to 72% styrene with focused sunlight irradiation.
The method's ability to depolymerize both pure and mixed plastic waste streams underscores its potential for large-scale application in plastic recycling.
Results Overview
- Synthesis and Depolymerization of PS-CB Composites:
- Polystyrene-carbon black composites (PS-CB) were synthesized with varying carbon black loadings (0.5 to 5 wt%).
- Under white LED light irradiation, the composites were depolymerized, yielding up to 57% styrene monomer at 5 wt% carbon black loading.
- The primary products included styrene monomer, dimer, trimer, toluene, and α-methylstyrene (AMS).
- Depolymerization of Post-Consumer Black Polystyrene:
- Unmodified post-consumer black polystyrene samples were successfully depolymerized without additional catalysts or solvents.
- Yields of up to 53% styrene monomer were achieved, demonstrating the method's effectiveness on real-world samples.
- Focused Solar Irradiation:
- Using focused solar irradiation (Fresnal Lens), the study achieved up to 80% styrene yield in just 5 minutes, showcasing the potential for high-efficiency depolymerization using renewable energy sources.
- Mixed Plastic Waste Depolymerization:
- The method was also effective in depolymerizing mixed-color post-consumer waste polystyrene samples, with styrene yields of up to 72% achieved using focused sunlight irradiation.
- Food Contaminants Screening:
- The presence of common food contaminants showed only slightly lower yields of 46% for samples containing 20% wt canola and soy sauce while sucrose remained unchanged.
Mechanistic Insights
The study proposes that depolymerization occurs via a combination of random chain scission and end-chain unzipping, where localized heating initiates bond breakage and monomer recovery. Gas chromatography-mass spectrometry (GC-MS) confirmed high selectivity toward styrene monomer formation, minimizing byproduct generation.
Conclusion
The research presents a novel and efficient method for recycling black polystyrene plastics using photothermal conversion with carbon black as a photothermal agent. The technique demonstrates high styrene monomer yields and practicality in real-world applications, offering a sustainable solution for plastic waste management. The integration of this method into existing recycling infrastructure could significantly reduce the environmental impact of plastic waste and promote a circular economy.

Revolutionizing Polystyrene Recycling Through Photothermal Conversion