Tooth loss is a widespread oral health issue that significantly impacts individuals' lives by affecting chewing, speaking, and overall well-being. Although current replacement methods like dentures and dental implants provide solutions, they often fall short of fully restoring the natural form and function of teeth.
Regenerative dentistry focuses on biological tooth replacement strategies, with tissue engineering emerging as a promising approach. A key challenge in this field is creating biomaterials that effectively support three-dimensional (3D) tooth organoid formation by mimicking the intricate developmental processes of natural teeth.
This research introduces defined bioorthogonally cross-linked hydrogels as novel 3D matrices for tooth developmental engineering, demonstrating the critical role their tunable properties play in enabling successful in vitro tooth organoid formation.
Key Takeaways
- Defined bio-orthogonally cross-linked hydrogels were successfully used for the first time as 3D matrices to engineer tooth organoids in vitro.
- The physicomechanical properties of these hydrogels, including elastic modulus (E) and storage modulus (G′), were precisely tuned by varying the gelatin concentration and the stoichiometric ratio of tetrazine (Tz) and norbornene (Nb) modified gelatin precursors.
- A specific hydrogel formulation (GEL_8%_R05) facilitated successful growth kinetics and morphogenesis of tooth germs derived from dental epithelial and mesenchymal cells.
- The study highlights the pivotal role of hydrogel properties in modulating the self-organization and interaction of dental cells, leading to the formation of functional tooth organoids.
- This research introduces a defined and tunable platform for tooth organoid engineering and modeling, holding significant potential for future applications in regenerative dentistry and the study of tooth development and related pathologies.
Overview
Regenerating a whole tooth through bioengineering requires replicating the complex interactions between dental epithelium and mesenchyme that occur during natural tooth development.
Pioneering work in 1987 demonstrated that interactions between specific embryonic cells could induce tooth formation, and subsequent in vitro studies showed that reassociated dental epithelial and mesenchymal cells from mouse embryos could self-organize into tooth germ-like structures.
These tissue-engineered constructs recreate developmental aspects of in vivo dental tissues and can develop into functional organs. They therefore qualify as organoids—3D in vitro structures derived from dental cells that replicate the developmental processes and structural complexity of natural teeth.
Achieving fully functional bioengineered teeth requires embedding appropriate cells within suitable 3D artificial matrices, known as biomaterials, that can support cell self-organization and promote proper tooth morphogenesis.
Various biomaterials, including polymers like polyglycolide acid (PGA), poly-L-lactide acid (PLLA), poly(lactic-co-glycolic acid) (PLGA), collagen sponges, and even decellularized tooth buds, have been explored.
Hydrogels—water-swollen polymer networks—have also been investigated for their ability to encapsulate cells in a 3D environment conducive to growth and rearrangement. However, many existing hydrogels, such as Matrigel, lack precise control over their physicomechanical properties, limiting our understanding of how these properties influence in vitro tooth organoid formation.
This research addresses this limitation by introducing defined bioorthogonally cross-linked hydrogels for tooth developmental engineering. The term bioorthogonal chemistry refers to chemical reactions that can occur within living systems without interfering with native biochemical processes.
The researchers utilized the click reaction between tetrazine (Tz) and norbornene (Nb) functionalized gelatin precursors (GEL_Tz and GEL_Nb) to form these hydrogels. By varying the gelatin concentration and the stoichiometric ratio of GEL_Tz to GEL_Nb, they could precisely tune the hydrogel's mechanical properties, including the elastic modulus (E), a measure of stiffness, and the storage modulus (G′), reflecting the material's ability to store energy and resist deformation.
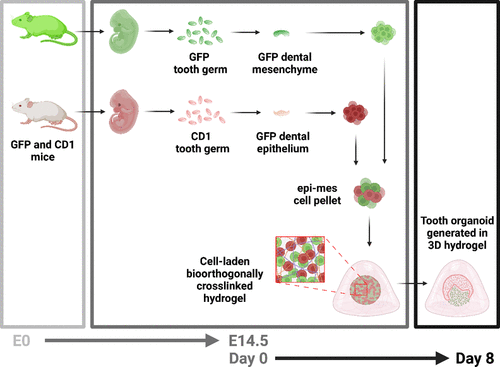
" Figure 1. Preparation of the 3D tooth organoids. Dental mesenchyme and epithelium were obtained in green fluorescent protein (GFP) and CD1 mouse embryos at embryonic day 14.5 (E14.5), separately. After digesting to single cell suspensions, the two cell populations were combined to obtain an epithelial-mesenchymal cell pellet, encapsulated in hydrogels (day 0), and cultured in vitro to generate 3D tooth organoids (day 8). Created with BioRender.com."
Why it’s Important
Tooth loss affects millions globally, and current treatments have significant limitations. This research paves the way for biological tooth replacement strategies by providing a controlled environment for creating tooth-like structures in vitro.
Understanding the intricate processes of tooth development, including the critical mesenchymal-epithelial crosstalk, is crucial for both regenerative efforts and for studying the pathogenesis of dental anomalies.
The tunable hydrogel platform developed in this study allows for systematic investigation of how the extracellular matrix (ECM), mimicked by the hydrogel, and its mechanical properties influence cell behavior and tissue morphogenesis during tooth development.
Previous studies have shown the potential of transplanted bioengineered tooth germs to develop into structurally correct teeth with vascularization and nerve fiber penetration from adult sources, suggesting the feasibility of autologous tooth regeneration, minimizing immunological rejection.
This in vitro system can serve as a valuable tool for studying the fundamental biology of organogenesis and for elucidating the mechanisms underlying developmental defects and diseases affecting teeth.
The ability to engineer functional tooth organoids that can potentially develop into mature teeth upon transplantation in vivo offers a promising regenerative therapy in lieu of current replacement techniques such as implants and crowns.
Summary of Results
The researchers successfully prepared bioorthogonally cross-linked gelatin hydrogels with tunable properties by mixing gelatin precursors modified with tetrazine (GEL_Tz) or norbornene (GEL_Nb). The degree of modification (DOM) for both GEL_Tz and GEL_Nb was quantified using 1H NMR spectroscopy.
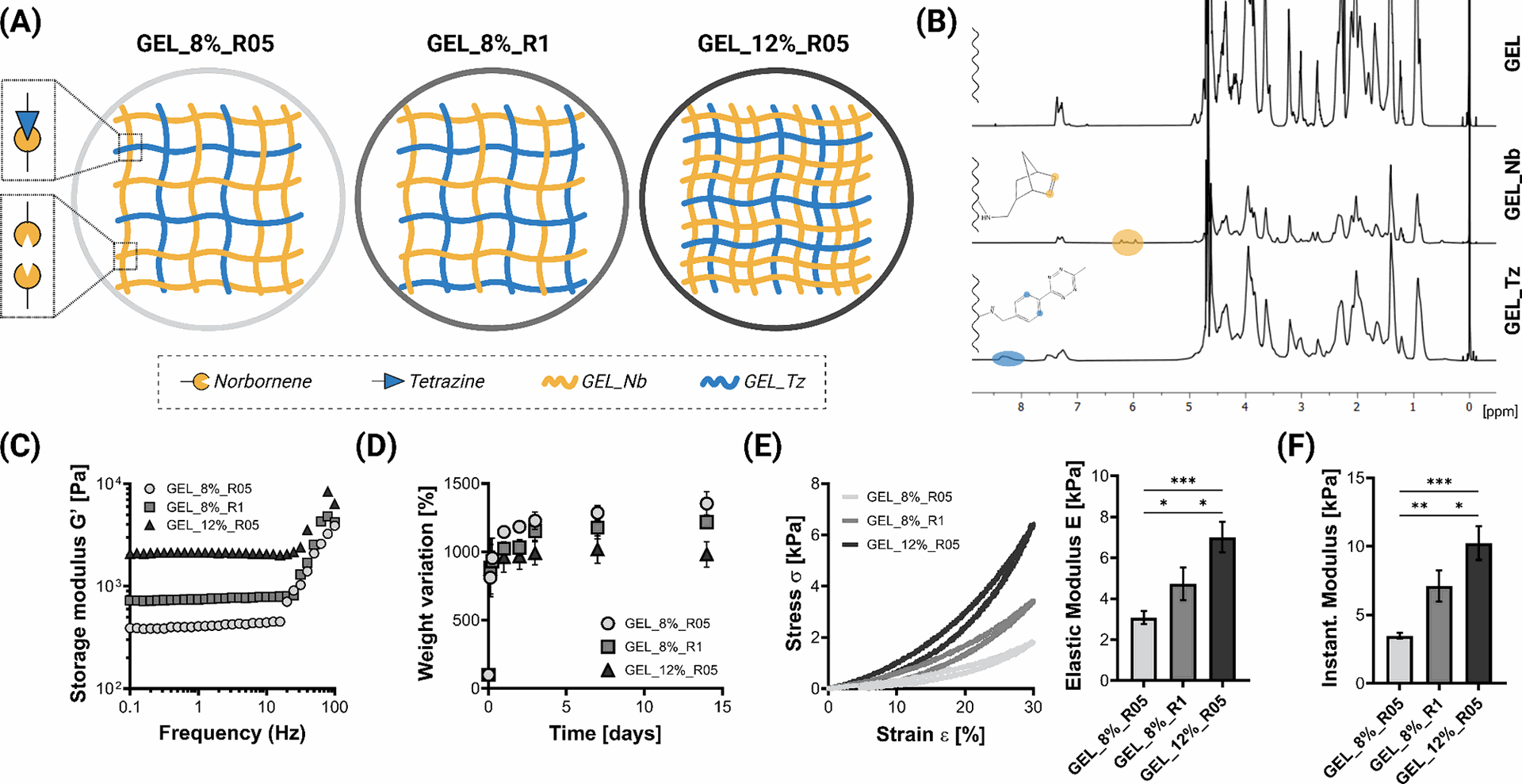
Figure 2 illustrates the preparation of these hydrogels with varying gelatin concentrations (8% and 12% w/V) and Tz:Nb stoichiometric ratios (1:1 denoted as R1 and 0.5:1 denoted as R05). Rheological tests confirmed hydrogel formation, showing that hydrogels with higher polymer concentration and stoichiometric ratio exhibited a higher storage modulus (G′). Swelling tests demonstrated the stability of all hydrogels in cell culture conditions for at least two weeks, with different swelling profiles observed for different formulations. Mechanical testing revealed that the elastic modulus (E) could be tuned between 2 and 7 kPa by adjusting the hydrogel concentration and stoichiometric ratio, with E increasing with higher concentration and a 1:1 Tz:Nb ratio. The hydrogels also proved to be cytocompatible, with cell viability exceeding 70% after one day of culture.
Dental mesenchyme and epithelium were obtained from mouse embryos, dissociated into single-cell suspensions, and combined into epithelial-mesenchymal cell pellets. These pellets were then encapsulated in the prepared hydrogels and cultured in vitro to generate 3D tooth organoids.
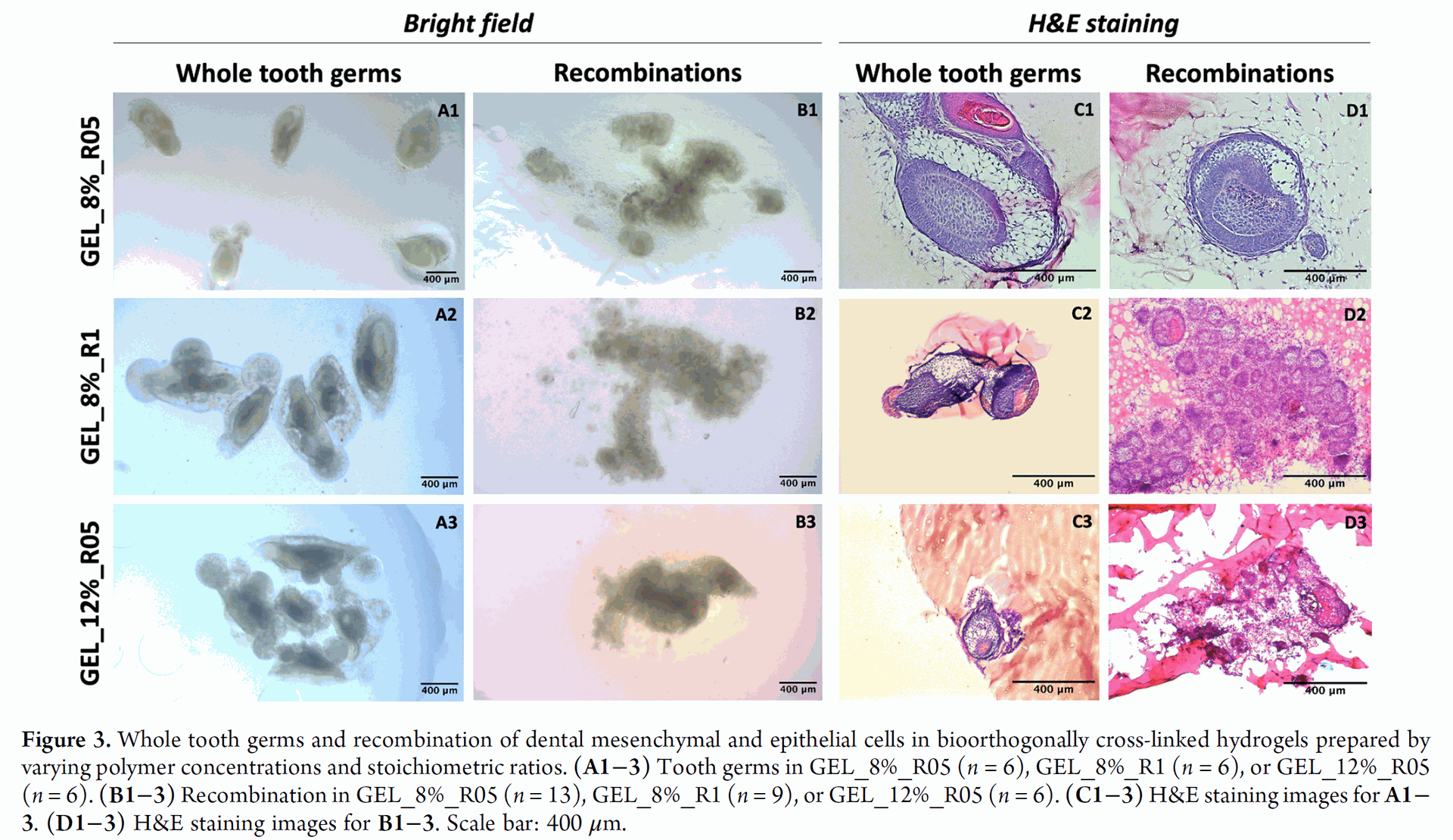
Figure 3 shows macroscopic images and H&E staining of whole tooth germs and recombined dental cells encapsulated in different hydrogel formulations (GEL_8%_R05, GEL_8%_R1, and GEL_12%_R05) after 8 days of in vitro culture.
The results indicated that hydrogel properties significantly influenced the structural progression of the encapsulated tooth germs. Higher cross-link density (GEL_8%_R1 vs. GEL_8%_R05) and higher gelatin concentration (GEL_12%_R05 vs. GEL_8%_R05) resulted in decelerated growth kinetics and changes in morphology. Tooth germs in lower stiffness hydrogels (e.g., GEL_8%_R05) displayed more typical structural development.
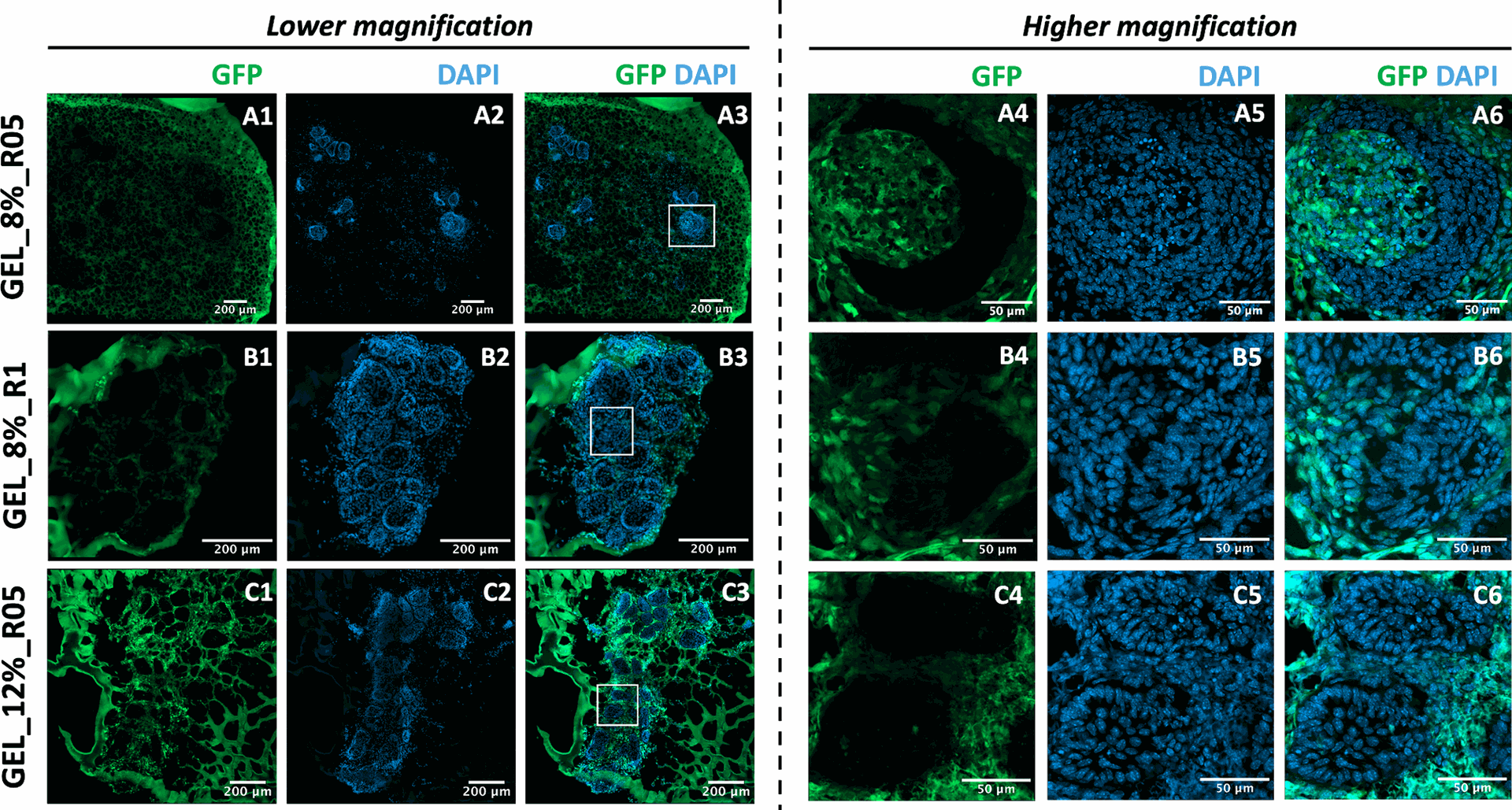
Figure 4 and accompanying videos (Supporting Information) further revealed the cellular composition and origin of cells within the tooth organoids using immunofluorescence. In the GEL_8%_R05 hydrogel, both epithelial (GFP-) and mesenchymal (GFP+) cells recombined and interacted dynamically, indicating successful dental organoid formation. In contrast, the other two hydrogel formulations (GEL_8%_R1 and GEL_12%_R05) did not support tooth organoid formation, as evidenced by the mesenchymal cells being located outside the epithelial structures, indicating a lack of communication.
These findings underscore that tuning the hydrogel properties is critical for modulating the occurrence of tooth organoids, providing valuable insights for tissue engineering and regenerative dentistry applications.
Conclusion
This research successfully demonstrated the generation of tooth organoids in vitro using defined bio-orthogonally cross-linked gelatin hydrogels.
By precisely controlling the hydrogel's physicomechanical properties the study identified a specific formulation (GEL_8%_R05) that effectively supports the self-organization and morphogenesis of dental epithelial and mesenchymal cells into tooth germ-like structures.
This work introduces a tunable platform for tooth organoid engineering and modeling, offering significant potential for advancing biological tooth replacement strategies in regenerative dentistry and for providing a controlled in vitro environment to study the fundamental mechanisms of tooth development and related pathologies.
The ability to use defined biomaterials with tailored properties represents a crucial step forward in achieving the goal of bioengineering functional teeth. Hopefully leading to a future where tooth replacement is done organically and permanently.

Advances in Lab Grown Tooth Replacement Techniques
Generating Tooth Organoids Using Defined Bioorthogonally Cross- Linked Hydrogels